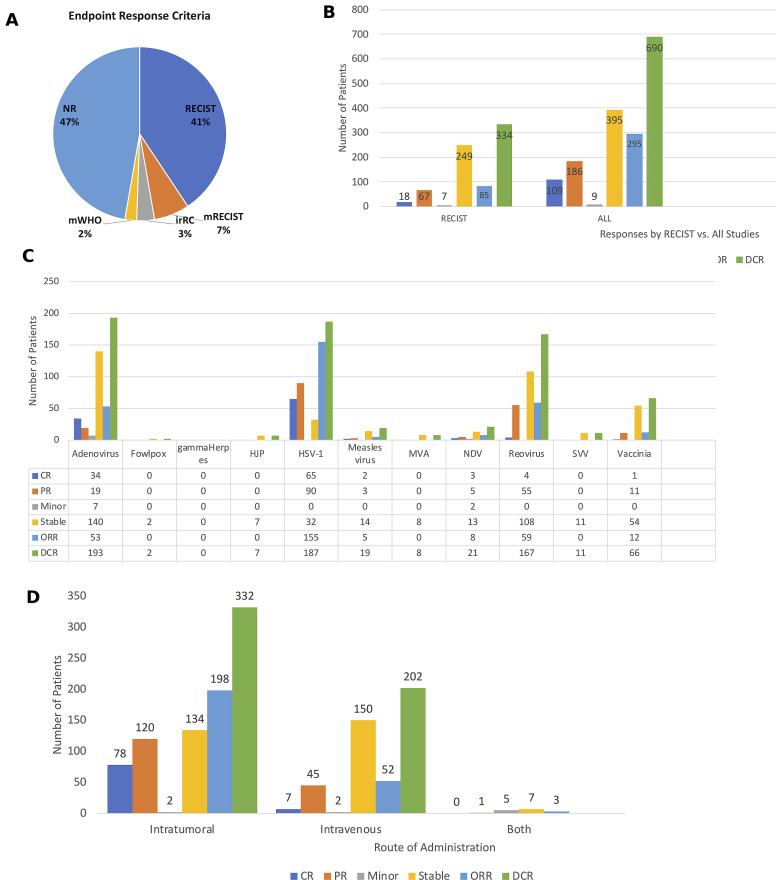
Figure 8.
Antitumor activity of oncolytic viruses (OVs) in clinical studies. (A) Pie chart showing the endpoint response criteria used to monitor clinical responses in the OV trials. (B) The number of patients with specific clinical responses in clinical trials using RECIST criteria (left panel) and in all studies (right panel). (C) Responses by type of OV used in the clinical study. (D) Responses by route of administration. Abbreviations: CR, complete response; DCR, disease control rate; irRC, immune-related RECIST criteria; Minor, minor response; mWHO, modified WHO criteria; NR, not reported; ORR, objective response rate; PR, partial response; RECIST, Response Endpoint Criteria in Solid Tumors; Stable, stable response.