Abstract
Background
This study aimed to investigate the therapeutic and prognostic effects of percutaneous transforaminal endoscopic decompression (PTED) for degenerative lumbar spinal stenosis (DLSS).
Material/Methods
One hundred eighty-eight patients with DLSS were randomly divided into the fenestration and the PTED group for decompression treatment. Operative time, incision length, amount of blood loss, length of hospitalization, and rates of complications in the 2 groups were compared. All patients underwent computed tomography (CT) scanning and magnetic resonance imaging (MRI) on the first postoperative day. All patients were assessed preoperatively and the treatment effects at 3, 6, and 12 months postoperatively were evaluated using visual analog scale (VAS), Japanese Orthopedic Association Score (JOA) and Oswestry Disability Index (ODI). The modified MacNab criteria were used to assess patient satisfaction 1 year after surgery at the last follow-up.
Results
Patients who underwent PTED had shorter incisions, less blood loss, and shorter hospital stays than those in the fenestration group, but operative times and complication rates were similar in the 2 groups. Moreover, CT scanning and MRI revealed similar treatment effects in the 2 groups. Compared with preoperative status, improvements in VAS, ODI, and JOA scores occurred at different times after surgery in the 2 groups. In particular, all 3 scores in the PTED group were higher than those in the fenestration group at 3 and 6 months postoperatively. There were no significant differences in MacNab scores between the 2 groups.
Conclusions
PTED is safer and more effective than traditional fenestration for management of DLSS.
MeSH Keywords: Fenestration, Labyrinth; Prognosis; Treatment Outcome
Background
Degenerative lumbar spinal stenosis (DLSS) is a disabling condition that is common in the elderly and is associated with a wide variety of symptoms, including low back pain, lower limb numbness, sensation disorders, weakness, bladder dysfunction, and claudication [1]. The precise incidence of degenerative spinal stenosis is unclear, but almost 50% of individuals over 60 years of age experience low back pain, which is commonly ascribed to DLSS [2]. Reported prevalence of DLSS in different regions varies from 20% to 60% [3–5]. Pathologically, degenerative changes in the vertebra, intervertebral disc or facet joints diminish available space for the neural and vascular elements in the lumbar spinal canal. Neurologic symptoms emerge when the narrowing finally impinges on the spinal cord or nerve roots [6].
There are many conservative therapies available for patients with mild stenosis, including epidural steroid injection, local anesthesia, soft tissue manipulation, exercise, spinal manipulation, use of braces and corsets, as well as pain-relieving treatments such as heat, ice, electrical stimulation and ultrasound [7]. However, patients with DLSS usually get limited relief of symptoms with these conservative approaches because their disease is of long standing, their compliance with treatment is poor, or they have relatively severe symptoms [8]. As a result, more and more patients with DLSS are seeking surgical interventions.
The traditional surgery for lumbar spinal stenosis is laminectomy, in which multiple structures in the spinal canal, including the spinous processes, vertebral lamina, ligamentum flavum, and parts of the facet joints, are removed [9]. Laminectomy is such invasive that it often results in iatrogenic instability and other secondary complications, such as infection and failure of internal fixation [10]. Thus, modified partial decompression techniques have been attempted, among which, fenestration is the most popular alternative to laminectomy. The procedure minimizes vertebral laminae resection with undercutting of the facetectomy and partial laminectomy, which may preserve vertebral stability and prevent dura adhesion [11].
Recently, minimally invasive techniques for spinal surgery have been described [12]. Image-guided minimally invasive techniques reportedly can enable physicians to relieve lumbar canal stenosis through percutaneous decompression of the hypertrophic ligamentum flavum [13]. With percutaneous transforaminal endoscopic discectomy (PTED), which requires only local anesthesia, patients with DLSS are operated on through a working cannula to which a camera is attached. Use of PTED reportedly costs less and results in fewer iatrogenic injuries and greater patient satisfaction [14,15]. However, more research is needed to evaluate the efficacy of PTED for management of DLSS. In the present study, we aimed to compare the prospective therapeutic and prognostic effects of PTED and fenestration decompression in patients with DLSS.
Material and Methods
Participants
This study included 188 patients with DLSS (98 men and 90 women, aged 65 to 85 years) hospitalized in Yangzhou Hongquan Hospital from April 2015 to May 2019. The inclusion criteria were: (1) clinical symptoms of neurogenic intermittent claudication; (2) diagnosis of DLSS with imaging; (3) ineffectiveness of conservative treatment for more than 3 months. The exclusion criteria were: (1) multiple segment lumbar stenosis; (2) narrowing of the vertebral canal or lateral recess; (3) intervertebral disc calcification; (4) lumbar spondylolisthesis; (5) lumbar trauma, cancer, severe osteoporosis, or rheumatoid arthritis and other systematic diseases. Patients were assigned by random figure table to either the PTED group or the fenestration group for decompression treatment. All participants provided written informed consent. This study was designed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Yangzhou Hongquan Hospital.
Surgical procedures
The fenestration decompression procedure was performed under general anesthesia with the assistance of a C-arm X-ray machine. A 3- to 4-cm posterior midline incision, centered over the lesion level, was made. Then the paravertebral muscles were split and retracted laterally to the outer edge of the facet joint. Then, in turn, the vertebral facet joints, adjacent lamina margin, and ligamentum flavum were resected.
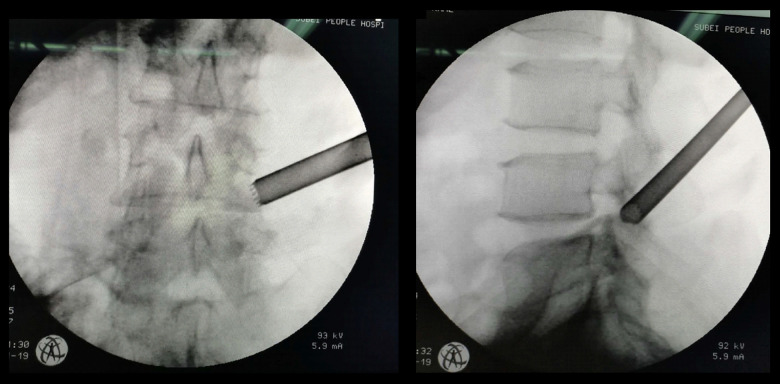
The PTED procedure was performed under local anesthesia with the assistance of a C-arm X-ray machine. A puncture was made 10 to 14 cm from the posterior middle line, along the superior facet joint and up to Kambin’s triangle of the neuroforamen. Kambin’s triangle is a three-dimensional anatomic right triangle over the dorsolateral intervertebral disk in the lumbar spine. A guidewire was inserted and a 0.7-cm incision was made. The dilated duct was located and the trephine was inserted. Then, the superior articular facet was gradually drilled to create a working channel. After X-ray confirmation that the working channel was appropriate (Figure 1), the transforaminal endoscopic surgical system (Joimax GmbH, Germany) was connected. Dorsal and ventral decompression of the nerve root was accomplished by removing the hypertrophied ligamentum flavum, the inferior part of the superior facet joint, and the upper and medial margins of the next vertebral pedicle. Decompression was confirmed when the nerve showed pulsations simultaneous with the patient’s heart rate. All patients received intravenous injections of flurbiprofen (50 mg/day) for postoperative analgesia.
Figure 1.
Working cannula position shown on an X-ray taken during percutaneous transforaminal endoscopic decompression. The working cannula was placed in a 65-year-old man.
Assessment of efficacy
In both groups, data on operative times, amount of blood loss, and postoperative complications were recorded. All patients underwent magnetic resonance imaging (MRI) and computed tomography (CT) scanning the day after surgery. A visual analog scale (VAS) with rankings from 0 (“no pain”) to 10 (“worst pain possible”), [16], the Japanese Orthopedic Association (JOA) score [16], and the Oswestry Disability Index (ODI) [17] were used to evaluate neurological function and pain levels in the patients preoperatively and 1 week and 3, 6, and 12 months postoperatively. The modified MacNab criteria were used to assess treatment efficacy 1 year after surgery, at the last follow-up visit.
Statistical analyses
All data were analyzed using SPSS 21.0 software (IBM Corp., Armonk, New York, U.S.A.). A Kolmogorov-Smirnov Goodness-of-Fit test was used to confirm that the data were normative. The data were presented as mean±standard deviation, and were compared using an independent t test. Enumeration data were analyzed using Fisher’s exact test. A two-way analysis of variance (ANOVA) test and Tukey’s range test were used for multiple comparisons. Two-tailed P values were chosen to evaluate significance and P<0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 shows that there were no significant differences between the 2 groups in sex, age (73.02±5.23 vs. 74.46±6.01), duration of symptoms (5.36±0.41 vs. 5.46±0.50), body mass index (BMI) (23.75±3.67 vs. 24.01±3.58), or level of stenosis (all P>0.05). These data confirm that the 2 groups had similar baseline characteristics.
Table 1.
Patient demographics in the fenestration and PTED groups.
| Characteristic | Fenestration (n=94) | PTED (n=94) | P |
|---|---|---|---|
| Age | 73.02±5.23 | 74.46±6.01 | 0.081 |
| Sex | 0.770 | ||
| Female | 44 (46.81%) | 46 (48.94%) | |
| Male | 50 (53.19%) | 48 (51.06%) | |
| Duration of symptoms (years) | 5.36±0.41 | 5.46±0.50 | 0.136 |
| BMI | 23.75±3.67 | 24.01±3.58 | 0.624 |
| Stenosis level | 0.725 | ||
| L3–L4 | 13 (13.83%) | 17 (18.09%) | |
| L4–L5 | 50 (53.19%) | 47 (50.00%) | |
| L5–S1 | 31 (32.98%) | 30 (31.91%) | |
| Comorbid disease (n/%) | |||
| Hypertension | 54 (57.45%) | 58 (61.70%) | |
| Diabetes | 26 (27.66%) | 29 (30.85%) | |
| Heart disease | 23 (24.47%) | 18 (19.15%) | |
| Cerebrovascular infarction | 13 (13.83%) | 15 (15.96%) | |
| Respiratory disease | 9 (9.57%) | 11 (11.70%) | |
| Renal/ureteral disease | 4 (4.26%) | 4 (4.26%) | |
| Peripheral vascular disease | 5 (5.32%) | 6 (6.38%) | |
| Neoplasia | 4 (4.26%) | 1 (1.06%) | |
| Anxiety neurosis | 2 (2.13%) | 1 (1.06%) | |
PTED – percutaneous transforaminal endoscopic decompression; BMI – body mass index. Sex and stenosis level were compared using Fisher’s exact test; the rest of the data were compared using a t test.
Clinical outcomes
All procedures in both groups were conducted successfully by the same surgical team. Compared with the fenestration group, the PTED group had significantly shorter incisions (3.57±0.75 vs. 0.82±0.32), less blood loss (115.32±11.46 vs. 49.63±5.86) and shorter hospital stays (2.55±0.71 vs. 4.62±0.83) (P<0.05). In the fenestration group, postoperative complications were 1 case of transient sensory disturbance and 2 cases of temporary pain aggravation, whereas the PTED group had 3 postoperative complications. No infections, thrombophlebitis, or cases of cauda equina syndrome or respiratory injury were seen in either group and the rate of mortality was 0%, as shown in Table 2. These indexes indicated the superiority of PTED when compared with fenestration decompression.
Table 2.
General clinical results in the fenestration and PTED groups.
| Clinical | Fenestration (n=94) | PTED (n=94) | P |
|---|---|---|---|
| Incision length (cm) | 3.57±0.75 | 0.82±0.32 | <0.001 |
| Blood loss (mL) | 115.32±11.46 | 49.63±5.86 | <0.001 |
| Operative time (min) | 66.32±15.32 | 68.75±11.56 | 0.085 |
| Hospital stay (days) | 2.55±0.71 | 4.62±0.83 | <0.001 |
| Complication rate | 3 (3.2%) | 3 (3.2%) | >1.000 |
PTED – percutaneous transforaminal endoscopic decompression. All data were compared using a t test.
Imaging results
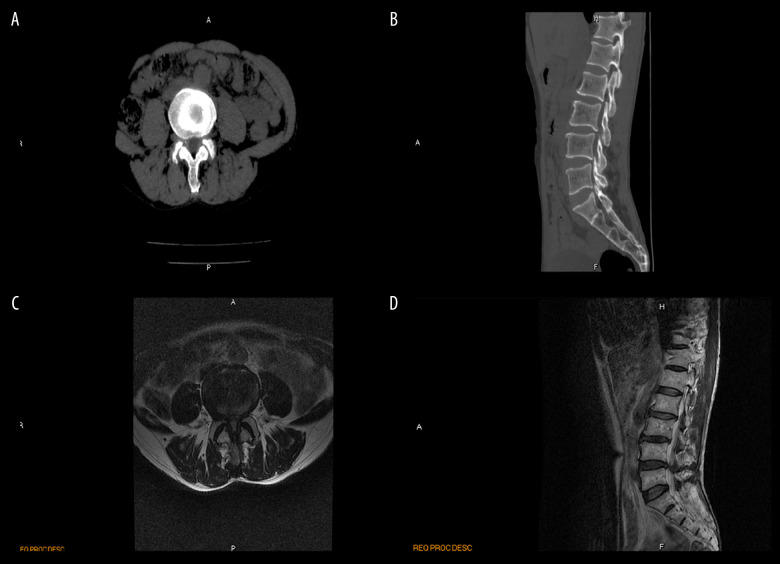
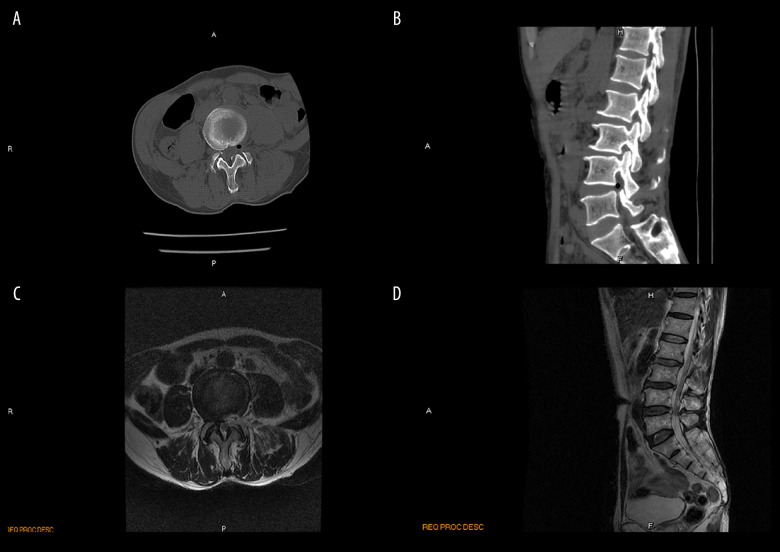
All patients underwent CT scanning and MRI 1 day before and 1 day after surgery. The images from a representative patient in the PTED group are described below. Before surgery, this patient had hyperplasia of the vertebral margins and lumbar degenerative changes. The lumbar physiology was curved. The L4–L5 disc bulged out, pressing the dural sac. Vertebral canal stenosis and hypertrophied ligamentum flavum were also seen at the L4–L5 level (Figure 2A–2D). After PTED, all of the patient’s lumbar segments became normal and although the physiological curvature was still present, the compression was gone (Figure 3A–3D).
Figure 2.
Preoperative computed tomography (CT) scan and magnetic resonance image (MRI) of a 65-year-old man with degenerative lumbar spinal stenosis. Preoperative CT (A, B) and MRI (C, D) showed vertebral canal stenosis and hypertrophied ligamentum flavum at the L4/L5 level (red arrowhead).
Figure 3.
Postoperative computed tomography (CT) scan and magnetic resonance image (MRI) taken during percutaneous transforaminal endoscopic decompression in a 65-year-old man with degenerative lumbar spinal stenosis. Compression is absent (red arrowhead) on the postoperative CT scans (A, B) and MRIs (C, D).
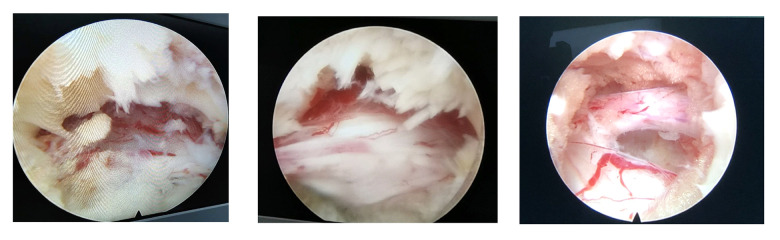
During PTED, nerve root congestion was observed. Removal of the hypertrophied ligamentum flavum relieved the compression (Figure 4).
Figure 4.
Images of the nerve root during percutaneous transforaminal endoscopic decompression in a 65-year-old man. The compression of the nerve root was relieved by removing the hypertrophied ligamentum flavum.
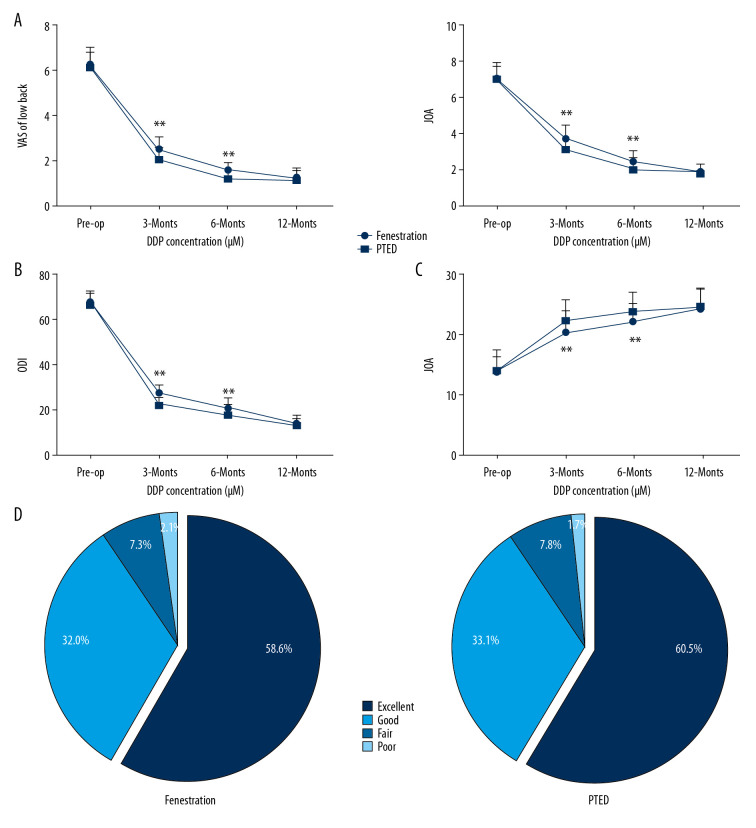
VAS, ODI, and JOA scores
Both groups had significant improvements in postoperative VAS and ODI scores compared with their preoperative statuses. Of note, compared with the fenestration group, the PTED group had better VAS, ODI, and JOA scores at 3 and 6 months postoperatively (P<0.01) (Figure 5A–5C). During the last follow-up visit, similar surgical outcomes were found in the 2 groups, based on McNab’s criteria (P>0.05) (Figure 5D). In addition, data were analyzed using repeated measurement of ANOVA. First, a Mauchly’s Test of Sphericity was performed and the P value was corrected for deviations using the Greenhouse-Geisser method. The results of the two-way ANOVA showed that the main outcomes of the operation mode were significant, but their interaction was not significant (Table 3).
Figure 5.
Comparison of postoperative treatment effects. (A) Visual analog scale, (B) Japanese Orthopedic Association Score, and (C) Oswestry Disability Index were assessed in the percutaneous transforaminal endoscopic decompression and fenestration groups preoperatively and postoperatively (1 week and 3, 6, and 12 months after treatment). (D) At the last follow-up visit (12 months after surgery), the modified Macnab criteria were applied to evaluate treatment effects. Data were analyzed using two-way ANOVA, followed by Tukey’s multiple comparisons test. ** P<0.01.
Table 3.
Results of two-way ANOVA.
| Variable | Source of variation | F | P |
|---|---|---|---|
| VAS of low back | Operation mode | 5.346 | 0.027* |
| Time | 3.017 | 0.040* | |
| Operation mode×time | 1.302 | 0.254 | |
| VAS of leg | Operation mode | 6.346 | 0.016* |
| Time | 7.238 | 0.000** | |
| Operation mode×time | 0.461 | 0.502 | |
| ODI | Operation mode | 2.808 | 0.033* |
| Time | 2.740 | 0.037* | |
| Operation mode×time | 0.250 | 0.833 | |
| JOA | Operation mode | 4.863 | 0.034* |
| Time | 6.632 | 0.001** | |
| Operation mode×time | 0.335 | 0.891 |
ANOVA – analysis of variance; VAS – visual analog scale; ODI – Oswestry Disability Index; JOA – Japanese Orthopedic Association.
Discussion
Patients with DLSS were treated with fenestration decompression or PTED to relieve their symptoms and then were followed for 1 year after the procedures. Figure 6 shows a flowchart of the present study. To the best of our knowledge, this is only the second report of outcomes with PTED for management of DLSS [8]. The results we have described showed that PTED was associated with less blood loss, shorter incisions, and shorter hospital stays than fenestration. The postoperative advantages of PTED that we observed are in keeping with other reports [16,18]. For elderly patients, a minimally invasive technique for DLSS that results in fewer iatrogenic injuries may result in faster recovery and easer rehabilitation [19]. Shorter hospital stays may be linked with lower risks of hospital-acquired infections, particularly in elderly patients who have weak immune systems [20]. With the increase of age, as is well known, incidence of DLSS, severity of relevant symptoms, and risks associated with anesthesia increase; therefore, the ability to perform PTED with only local anesthesia makes the procedure attractive.
Figure 6.
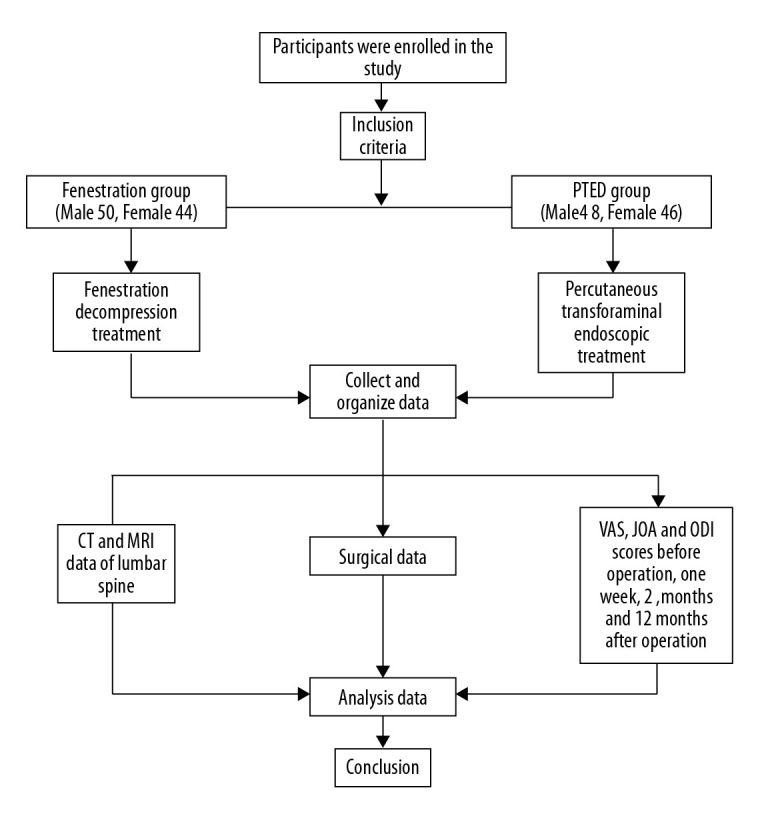
Flowchart of the present study. One hundred eighty-eight patients met the eligibility criteria for degenerative lumbar spine stenosis and were assigned by random figure table to either the percutaneous transforaminal endoscopic decompression group or the fenestration group. Incision length, amount of intraoperative blood loss, length of procedure and hospitalization, and complication rates in the 2 groups were compared. In addition, computed tomography scanning and magnetic resonance imaging were performed. Finally, scores from a visual analog scale, the Japanese Orthopedic Association Score, and the Oswestry Disability Index were collected before and at 3, 6, and 12 months after the procedures. The modified Macnab criteria were applied to assess patient satisfaction at the last follow-up, 1 year after the procedures, and the data were analyzed to draw a conclusion.
Although postoperative clinical outcomes at 12 months were comparable between the 2 groups, we did note better improvement in 3- and 6-month VAS, ODI, and JOA scores in patients who underwent PTED than those in patients who underwent fenestration decompression. The prognosis after PTED appeared to be better over the short term than over the longer term. One possible explanation for this phenomenon is that body physiological functions were restored more quickly in patients in the PTED group than those in the fenestration group because surgery resulted in less damage. Consistent with this, a previous study showed that PTED was associated with better postoperative ODI scores and shorter procedures and hospitalizations than fenestration discectomy for treatment of lumbar disc herniation [21]. Biportal endoscopic spinal surgery (BESS) unilateral laminotomy bilateral decompression (ULBD) also is associated with hospital stays similar to those with microscopic ULBD [22].
The present study has some limitations. First, all of the patients were treated at a single center, which limits the sample size and resulted in a non-representative profile of research participants. Secondly, it took 4 years to recruit 188 participants, which may lead to potential heterogeneity in the procedures that the individual patients underwent, because biases may have arisen from time-dependent improvements in the equipment used and the skills of our surgical team. Thirdly, individual differences in patients may be a very important factor to consider when studying senescence-related conditions. Unfortunately, in the present study, it was difficult for us to pair participants based on demographic characteristics, disease indexes, or socioeconomic factors or to perform extra subgroup analyses. In the future, we plan to explore the performance of PTED for DLSS with better-designed trials and larger samples. Last but not least, the data we presented only reflect 1-year prognosis, which is a relatively short period. We are continuing to follow the patients in the present study and more indicators will be observed.
Conclusions
In summary, our findings indicate that both fenestration and PTED are safe for management of DLSS, but the latter procedure has better short-term clinical effects. Because normalizing use of PTED in clinical practice could be beneficial for elderly patients, more studies of it should be carried out to further assess the safety and curative effects of the procedure for DLSS.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Abbas J, Peled N, Hershkovitz I, Hamoud K. Is lumbosacral transitional vertebra associated with degenerative lumbar spinal stenosis? Biomed Res Int. 2019;2019 doi: 10.1155/2019/3871819. 3871819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritz JM, Rundell SD, Dougherty P, et al. Deconstructing chronic low back pain in the older adult-step by step evidence and expert-based recommendations for evaluation and treatment. Part VI: Lumbar spinal stenosis. Pain Med. 2016;17(3):501–10. doi: 10.1093/pm/pnw011. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403–8. [PubMed] [Google Scholar]
- 4.Haig AJ, Tong HC, Yamakawa KS, et al. Spinal stenosis, back pain, or no symptoms at all? A masked study comparing radiologic and electrodiagnostic diagnoses to the clinical impression. Arch Phys Med Rehabil. 2006;87(7):897–903. doi: 10.1016/j.apmr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Ishimoto Y, Yoshimura N, Muraki S, et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthritis Cartilage. 2012;20(10):1103–8. doi: 10.1016/j.joca.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Sakai Y, Yamazaki K, et al. Relationship between L4/5 lumbar multifidus cross-sectional area ratio and fall risk in older adults with lumbar spinal stenosis: A retrospective study. Geriatrics (Basel) 2019;4(2):38. doi: 10.3390/geriatrics4020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi: 10.1136/bmj.h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Z, Jin L, Wang K, et al. Comparison of effects of PELD and fenestration in the treatment of geriatric lumbar lateral recess stenosis. Clin Interv Aging. 2019;14:2187–94. doi: 10.2147/CIA.S226295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thome C, Borm W, Meyer F. Degenerative lumbar spinal stenosis: Current strategies in diagnosis and treatment. Dtsch Arztebl Int. 2008;105(20):373–79. doi: 10.3238/arztebl.2008.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleeman TJ, Hiscoe AC, Berg EE. Patient outcomes after minimally destabilizing lumbar stenosis decompression: The “Port-Hole” technique. Spine (Phila Pa 1976) 2000;25(7):865–70. doi: 10.1097/00007632-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Li ZZ, Hou SX, Shang WL, et al. Percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar decompression for lateral recess stenosis through transforaminal approach: Technique notes and 2 years follow-up. Clin Neurol Neurosurg. 2016;143:90–94. doi: 10.1016/j.clineuro.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Deer TR, Kapural L. New image-guided ultra-minimally invasive lumbar decompression method: The mild procedure. Pain Physician. 2010;13(1):35–41. [PubMed] [Google Scholar]
- 14.Seiger A, Gadjradj PS, Harhangi BS, et al. PTED study: Design of a non-inferiority, randomised controlled trial to compare the effectiveness and cost-effectiveness of percutaneous transforaminal endoscopic discectomy (PTED) versus open microdiscectomy for patients with a symptomatic lumbar disc herniation. BMJ Open. 2017;7(12):e018230. doi: 10.1136/bmjopen-2017-018230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Dou Q, Yang J, et al. Percutaneous endoscopic lumbar decompression for lumbar lateral spinal canal stenosis: Classification of lateral region of lumbar spinal canal and surgical approaches. World Neurosurg. 2018;119:e276–83. doi: 10.1016/j.wneu.2018.07.133. [DOI] [PubMed] [Google Scholar]
- 16.Bao BX, Zhou JW, Yu PF, et al. Transforaminal endoscopic discectomy and foraminoplasty for treating central lumbar stenosis. Orthop Surg. 2019;11(6):1093–100. doi: 10.1111/os.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daltroy LH, Cats-Baril WL, Katz JN, et al. The North American spine society lumbar spine outcome assessment Instrument: Reliability and validity tests. Spine (Phila Pa 1976) 1996;21(6):741–49. doi: 10.1097/00007632-199603150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Depauw P, Gadjradj PS, Soria van Hoeve JS, Harhangi BS. How I do it: Percutaneous transforaminal endoscopic discectomy for lumbar disk herniation. Acta Neurochir (Wien) 2018;160(12):2473–77. doi: 10.1007/s00701-018-3723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Kong W, Liao W, et al. Percutaneous total endoscopic resection of partial articular processes for treatment of lateral crypt stenosis and lumbar spinal stenosis: Technical report and efficacy analysis. Biomed Res Int. 2018;2018 doi: 10.1155/2018/9130182. 9130182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrasa-Villar JI, Aibar-Remon C, Prieto-Andres P, et al. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644–52. doi: 10.1093/cid/cix411. [DOI] [PubMed] [Google Scholar]
- 21.Ding W, Yin J, Yan T, et al. Meta-analysis of percutaneous transforaminal endoscopic discectomy vs. fenestration discectomy in the treatment of lumbar disc herniation. Orthopade. 2018;47(7):574–84. doi: 10.1007/s00132-018-3528-5. [DOI] [PubMed] [Google Scholar]
- 22.Pranata R, Lim MA, Vania R, July J. Biportal endoscopic spinal surgery versus microscopic decompression for lumbar spinal stenosis: A systematic review and meta-analysis. World Neurosurg. 2020;138:e450–58. doi: 10.1016/j.wneu.2020.02.151. [DOI] [PubMed] [Google Scholar]