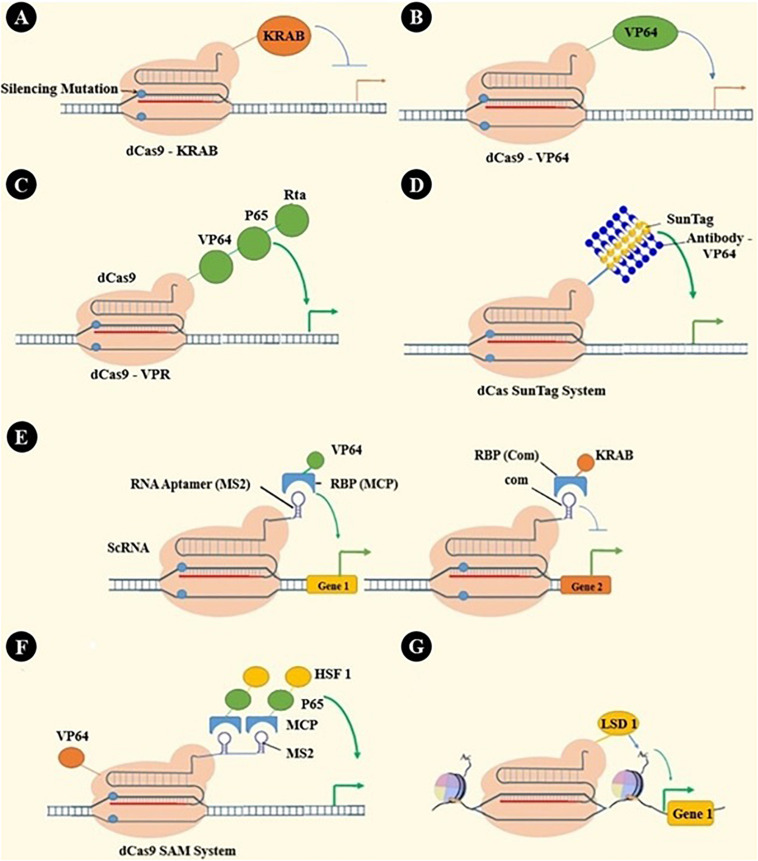
FIGURE 3.
dCas applications: beyond genome editing. (A) dCas9-KRAB has been engineered by fusing the KRAB transcription repressor domain to dCas9 (201). (B) VP64, as a transcription activator, has been fused to dCas9 to activate a specific gene’s transcription (151, 152, 202, 203). (C) Recruiting multiple transcriptional activators shows synergistic effects that have led to engineering many systems, such as VPR by in-tandem fusing of both p65 and Rta to VP64 (204). (D) SunTag is a repeating protein-peptide array that recruits multiple antibody-fusion proteins. Protein domains, such as transcriptional activating or epigenetic modulating domains, can be recruited by antibody-mediated binding to SunTag, which is fused with dCas (205, 206). (E) RNA aptamers (e.g., MS2, com, PP7) can be fused with sgRNA to create a scaffold RNA (scRNA) that can recruit RNA-binding proteins (RBPs, e.g., MCP, Com, PCP). Fusing each RBP to the effector protein has enabled gene activation, repression, or even simultaneous activation and repression in one cell (207). (F) In the synergistic activation mediator (SAM) system, effectors are recruited by both dCas and scRNA (208). (G) Epigenetic modifying enzymes like P300 and LSD1 can be fused with dCas and alter cells’ epigenomic features. These alterations were locus-specific epigenetic editing, including histone modifications and DNA methylations (205, 209, 210).