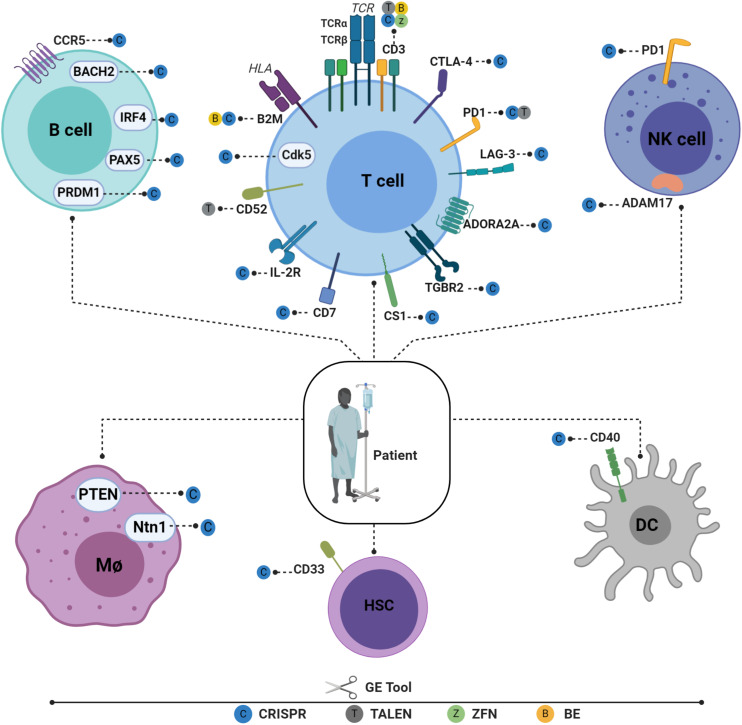
FIGURE 2.
Recent advances in engineering different immune cell types for immunotherapy applications. Engineered T cells in the B2M gene have lowered HLA class I antigen expression on the cell surface and have reduced the possibility of graft rejection. TCR/CD3 cells have been knocked out to reduce GVHD and to enable physiological CAR expression, thus enhancing CAR T potency. Tumor-suppressive microenvironments have been overcome by downregulating CTLA-4, PD1, and LAG-3. T cells have also been engineered to ignore suppressive signals by expressing dominant negative TGF beta receptors (TGBR2). On the other hand, to engraft T cells under lymphodepleting preparative conditions, the elimination of CD52 is required to enable T cells to resist alemtuzumab-mediated lymphodepletion. Targeting CD7 prevents fratricide and enables the expansion of CD7 CAR T cells without compromising their cytotoxic function. IL-2R has also been engineered to facilitate IL12P70 expression in a controlled manner. Cyclin-dependent kinase 5 (Cdk5), a serine/threonine kinase, whose inhibition confers antitumor immunity, has been identified to regulate the PD-1/PD-L1 pathway. HSPCs are also gene edited to enhance adoptive immunotherapy. CD33-deficient human HSPCs resistant to CD33-targeted approaches have been produced to mitigate CART33 toxicity, to maintain myelopoiesis, and to prevent on-target off-tumor toxicity. Immune NK cells play an important role in host immunity against cancer and viral infections. Despite the low efficiency of viral and non-viral delivery methods, several NK cells can be edited to enhance their persistence, cytotoxicity, and tumor targeting (117). Dendritic cells (DCs) play a critical role in T-cell response instructions, with triple knockout established as proof of concept (118). A similar approach is used to target the costimulatory molecule CD40, whose disruption significantly inhibits T-cell activation, thus reducing graft damage and prolonging graft survival (88). Macrophages can also be edited using CRISPR-CAS9 by targeting USP7 and USP47, two genes that regulate inflammasome activation (119). The Ntn1 gene, thought to be involved in cell migration disruption, can also be targeted in vivo using nanoparticles encapsulating CRISPR/Cas9 RNPs under the control of the CD68 promoter (89). To elucidate its role in inflammation, the NEU1 gene can be targeted in macrophages using CRISPR-CAS9, thus demonstrating the role of NEU1 macrophages as inflammation enhancers (120). It is also possible to engineer B cells to express mature broadly neutralizing bNAb antibodies targeting IgH loci or safe harbor CCR 5 in the case of FIX. Figures were created by BioRender.com.