Figure 4.
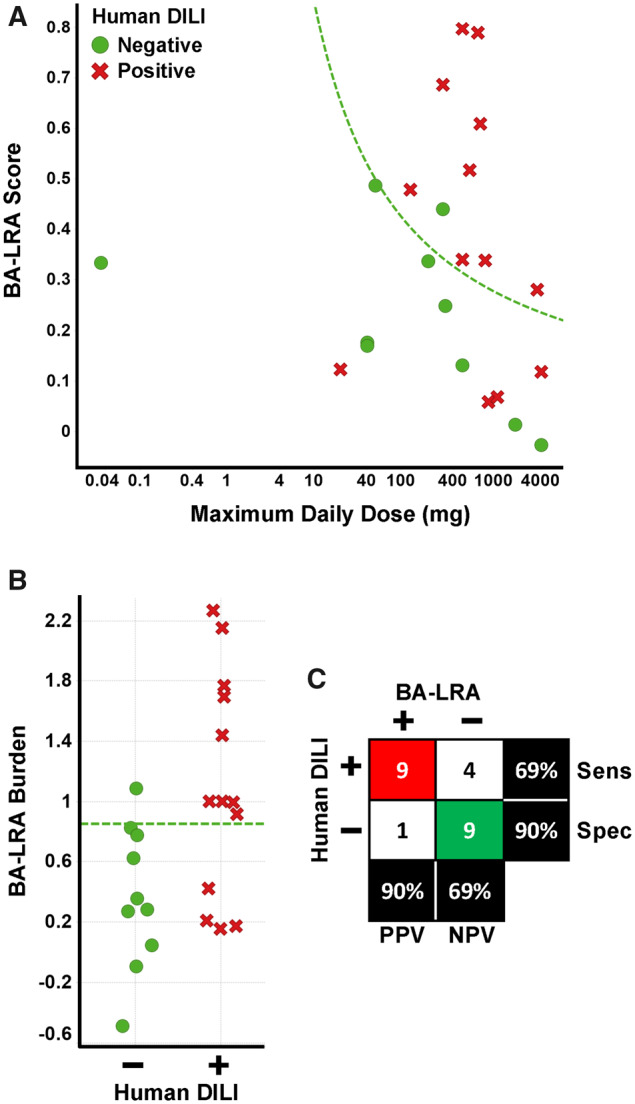
Consideration human daily dose improves performance of liver response score. A, The relationship of bioactivation liver response assay (BA-LRA) score and maximum daily clinical dose for the 23 compounds sufficiently tested and without evidence of signature uncoupling. Green circles indicate compounds with a lack of evidence for liver injury in humans, whereas red x’s indicate compounds with the ability to cause liver injury in humans. Dashed green line indicates BA-LRA burden (BA-LRA score × maximum daily clinical dose) threshold of 0.85. B, The BA-LRA burden (BA-LRA score × maximum daily clinical dose based) is plotted for the same compounds in (A). Compounds with a lack of (−) or ability (+) to cause liver injury in humans are plotted in green and red, respectively. Dashed green line indicates a BA-LRA burden threshold of 0.85. C, Matrix (2 × 2) of the association of drug-induced liver injury (DILI) risk and BA-LRA burden > 0.85. Red highlights number of true positives, green the number of true negatives. Abbreviations: Sens, sensitivity; Spec, specificity.
