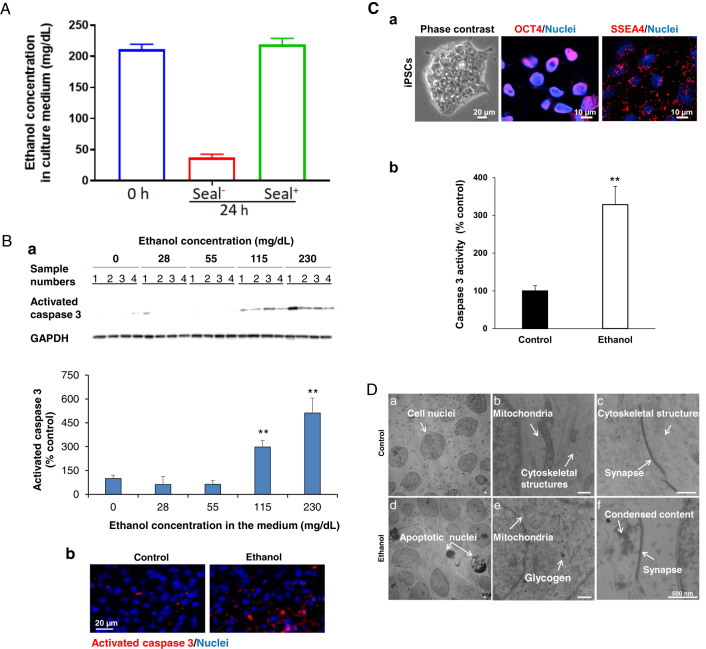
Fig. 2. Characterization of ethanol-induced apoptosis and ultrastructure changes in cerebral organoids derived from human iPSC line 1 (B and D) and line 2 (C).
A Analysis of ethanol concentration in culture medium over 24-h ethanol (230 mg/dL) exposure using Alcohol Assay Kit. The culture dishes were sealed with Parafilm™ during the ethanol treatment to prevent ethanol evaporation. Alcohol maintained the same concentration in culture medium over 24-h ethanol exposure. However, ethanol concentration dramatically decreased in the non-sealing dishes (n = 3). B Ethanol exposure dose-dependently induces apoptosis in iPSC-derived cerebral organoids. Two-month human iPSC line 1-derived cerebral organoids were treated with increasing concentrations of ethanol (0, 28, 44, 115, and 230 mg/dL). Western blot assay showed that ethanol dose-dependently increased the expression of activated caspase 3 (an apoptotic cell marker) in iPSC-derived organoids compared to the control group (B-a). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal housekeeping gene for normalization of activated caspase 3 expression data in the Western blot assay. Data are presented as mean ± standard error of the mean (SEM), n = 4, **P < 0.01 vs. non-ethanol treatment control. Immunofluorescence staining confirms that ethanol (230 mg/dL) treatment increased activated caspase 3-positive apoptotic cells (red) in the organoids. Nuclei were stained in blue (B-b). Scale bar = 20 μm. C Human iPSC line 2 grew as colonies in the culture and expressed pluripotent stem cell markers OCT4 (red) and SSEA4 (red). Nuclei were stained with Hoechst 33342 in blue. Scale bar = 20 or 10 µm (a). Ethanol (230 mg/mL, 6 h) induces apoptosis in human iPSC line 2-derived cerebral organoids (b). n = 4, *P < 0.05 vs. non-ethanol treatment control. D Electron microscopy images of apoptosis, cellular and subcellular alterations of cells in control (D-a to c), and ethanol (230 mg/mL, 6 h)-treated cerebral organoids (D-d to f). In the control organoids, cells appeared healthy with normal nuclei (D-a), normal mitochondria (D-b), and well-organized cytosolic structure. Cytoskeletal structures appeared to be present and looking normal (D-b and c) around nuclei and synapse (D-c). Well organized and homogenous cellular content was also observed. In the ethanol-treated organoids, apoptotic dark nuclei, condensed and fragmented chromatin appeared in the shrunken apoptotic cells. Formation of classic apoptotic ‘half-moon’ nuclear morphology bodies was seen in some cells (D-d). Abnormal mitochondria with less dense matrix and disrupted cristae were commonly observed together with abundant glycogen compared with control cells (D-e). There appeared to be disruption of the cytoskeleton with components not being visible within cells and the presence of condensed content around synapse (D-f). Scale bar = 500 nm.