Figure 1.
Models of Translational Research
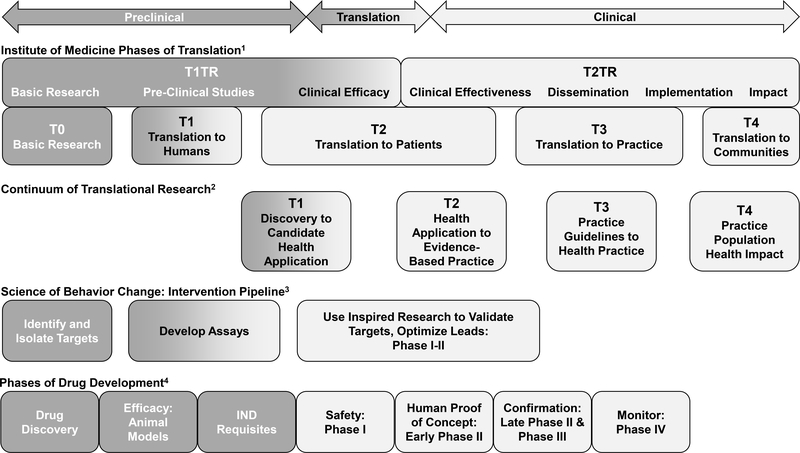
Four models of translational research reviewed in the manuscript are displayed to highlight their overlap and opportunities for translational and reverse translational research. The Institute of Medicine1 classifies research in two halves: Type 1 Translational Research (T1TR) which focuses on basic research to clinical efficacy, and Type 2 Translational Research (T2TR) emphasizes clinical efficacy, dissemination, implementation, and impact (Lam et al., 2013). The continuum of translational research2 includes four phases beginning with the identification of targets to candidate health application (T1) and ending with the evaluation of real-world health outcomes in the population (T4) (Khoury et al., 2007). The Science of Behavior Change proposed intervention pipeline3 highlights the identification of intervention targets, the development of appropriate assays, and the optimization of trial designs to measure target engagement (Nielsen et al., 2018). Finally, the Phases of Drug Development represents the pathway from drug discovery and pre-clinical animal research through translation to patient populations and safety monitoring of approved medications (Litten et al., 2012).
Phases of preclinical research are colored in dark gray, phases which overlap preclinical and clinical research, termed translation, are colored in a gradient from dark to light gray, and clinical research phases are colored in light gray.