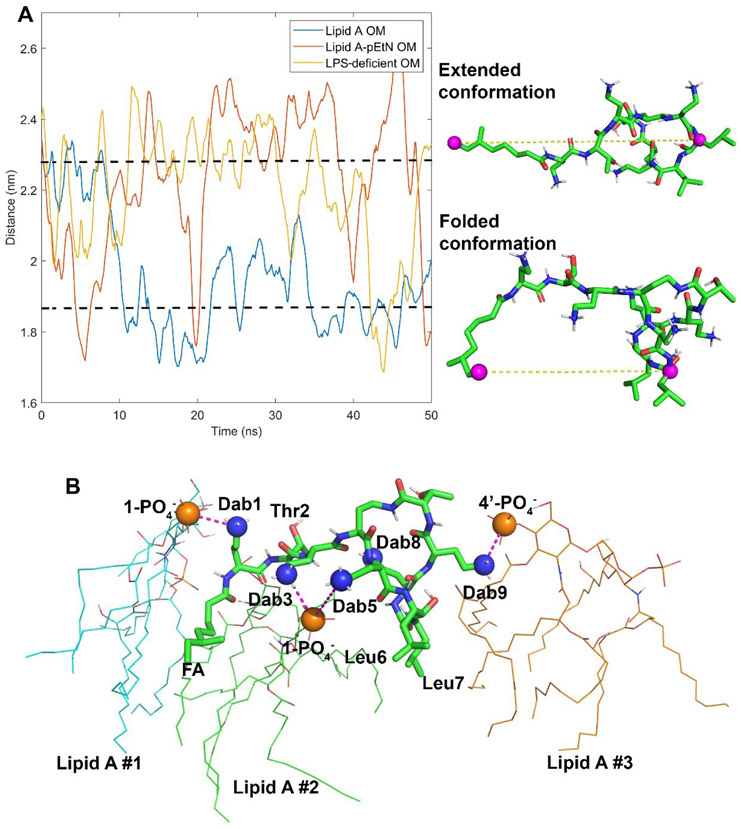
Figure 5.
Conformational dynamics of the colistin molecule during outer membrane (OM) penetration. (A) The last carbon atom of fatty acyl tail and the α-carbon atom of d-Leu6 are shown in the colistin structure with purple spheres. The distances between these two atoms are used to describe the conformational dynamics of the colistin molecule in different OMs. (B) The interaction model of the colistin molecule with the lipid A OM. The electrostatic interactions between the side chains of the Dab residues and the lipid A phosphate groups are shown with purple dashed lines.