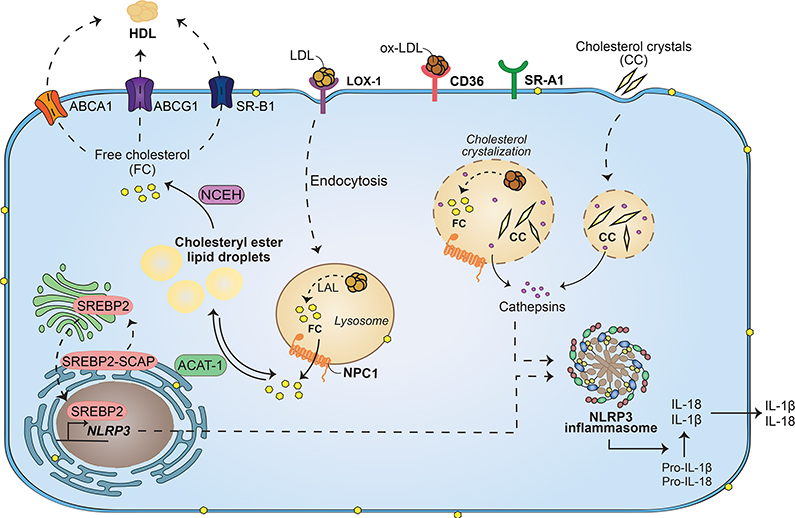
Figure 2. Macrophage lipid metabolism and links to NLRP3 inflammasome activation.
Aside from the uptake of LDL via LDL-R, macrophages take up modified lipids such as oxLDL via scavenger receptors including CD36, SR-A1, and LOX-1. The normal fate of cholesteryl esters in these lipoproteins transits through the lysosome where hydrolysis via LAL liberates free cholesterol. Lysosomal NPC1 shuttles free cholesterol out of the lysosome for re-esterification by ACAT-1 and subsequent storage in lipid droplets. Neutral lipid hydrolysis by cytoplasmic hydrolases can then re-liberate this cholesterol for efflux out of macrophages by ABCA1, ABCG1, or SR-B1 to HDL, thereby constituting the initial events in reverse cholesterol transport. Depending on intracellular cholesterol levels, endogenous cholesterol biosynthetic mechanisms involving SREBP2 can also be initiated. Each of the steps in cholesterol handling by macrophages can interact with or stimulate the NLRP3 inflammasome. Excessive oxidized lipid uptake and inefficient hydrolysis/shuttling in lysosomes can favor in situ formation of cholesterol crystals in lysosomes. Extracellular uptake of cholesterol crystals contributes to this overload. Both events compromise lysosomal membranes leading to inflammasome activation. Activation of SREBP2 can transcriptionally upregulate components of the inflammasome including NLRP3 providing increased machinery for IL-1β and IL-18 generation.
Abbreviations: LDL (low-density lipoprotein), LDL-R (low-density lipoprotein receptor), CD36 (cluster differentiation 36), SR-A1 (class A1 scavenger receptor), and LOX-1 (lipoxygenase-1), NPC1 (Niemann-Pick disease, type C1), ACAT-1 (acetyl-coenzyme A acetyltransferase 1), ABCA (ATP Binding Cassette Subfamily A Member 1), ABCG1 (ATP Binding Cassette Subfamily G Member 1), SR-B1 (class B1 scavenger receptor), HDL (high-density lipoprotein), SREBP2 (sterol response element binding protein 2)