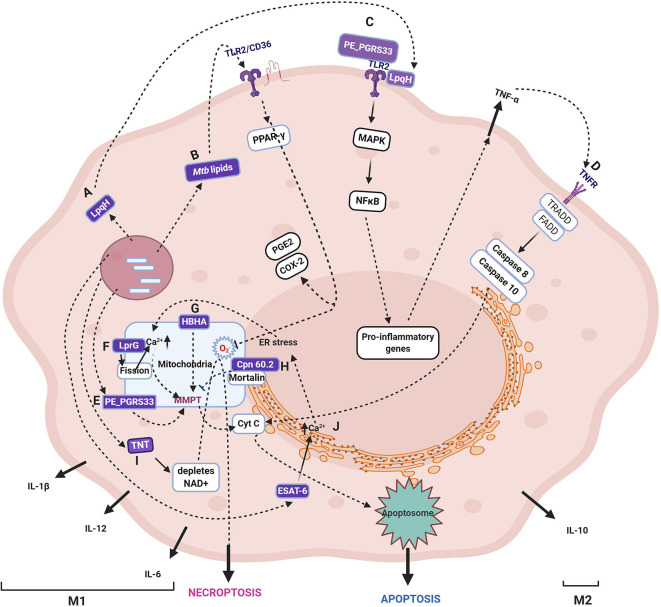
Figure 2.
Schematic representation of various cell fates upon Mycobacterium tuberculosis infection. The figure depicts the Mtb proteins that are demonstrated to translocate to mitochondria and their role in induction or inhibition of cell death modalities. The proteins, lipids, and carbohydrates that are either secreted or on the surface of Mtb are recognized by host pattern recognition receptors (majorly TLR2). The several proteins secreted by Mtb target various host organelles, of which mitochondria is a crucial target. Specific Mtb proteins, as discussed in the text, are reported to translocate to the mitochondrial membrane, wherein they cause a change in MMP, leading to Cytochrome C release and, subsequently, apoptosis. The specific pathway differs for each Mtb protein with differences in the source of perturbation, such as the generation of ROS, changes in Ca2+ dynamics, and mitochondrial fission. Some proteins are not reported to translocate to mitochondria, but their activities ripple their effect on mitochondria, such as TNT, which causes NAD+ depletion leading to increased ROS generation in mitochondria. The receptors, including TLR, TNF, IL-1R, NOD, signal through various combinations of adaptor proteins that result in specific signaling pathways and diverse outcomes such as apoptosis, necrosis, necroptosis, and autophagy as described in the text. The figure depicts the Mtb proteins that are demonstrated to translocate to mitochondria and their role in the induction or inhibition of cell death modalities. One of the several Mtb proteins that inhibit apoptosis by interacting with mitochondrial mortalin is Cpn60.2. (H). LpqH (A) and PE_PGRS33 (D) interact with TLR2 (C) and induces apoptosis through TNF-α. In the intracellular milieu, Mtb factors upon translocation to mitochondria and causes a change in MMP and leads to apoptosis (E). Mtb lipids such as Man-LAM can bind to TLR2/CD36 and signal PPAR- γ resulting in proinflammatory cytokines (B). HBHA is another secreted Mtb protein that causes apoptosis by changing MMP (G). LprG causes changes in Ca2+ dynamics and mitochondrial fission that leads to MMPT and, thus, apoptosis (F). One of the secreted proteins, TNT, hydrolyzes NAD resulting in the accumulation of ROS, and finally, necroptosis (I). The major secreted virulent protein, ESAT-6, causes ER stress that is transferred to mitochondria and finally culminating in apoptosis (J). It may be noted that the early phase of infection is dominated by either apoptosis that leads to bacterial clearance or anti-apoptosis that promotes bacterial survival and growth (M1). Once Mtb survives the host pro-inflammatory responses, it takes over by modulating the host metabolism by anti-inflammatory cytokines and alterations in lipid metabolism that inhibit apoptosis and autophagy coinciding with the M2 phase. As cell death is one of the major outcomes of perturbation in mitochondrial activities with or without getting targeted by mycobacterial factors, for a comprehensive list of cell death modulators from Mtb, please refer (Mohareer et al., 2018). The mycobacterial proteins are marked in purple boxes. The figure has been made using the Biorender app.