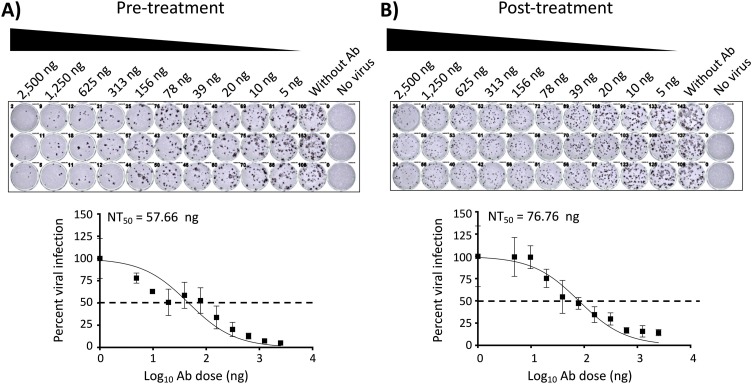
Fig. 2.
PRMNT assay to identify SARS-CoV-2 NAbs, peroxidase staining. A) Pre-treatment: A 2-fold serially diluted NAb-containing sample was pre-incubated with SARS-CoV-2 (∼100-200 PFU/well) for 1 h at 37°C. After 1 h pre-incubation, confluent monolayers of Vero E6 cells were then infected with the sample-virus mixture for 1 h at 37°C. After 1 h of virus adsorption, the inoculum was removed and replaced with post-infection media containing 1% Avicel. B) Post-treatment: confluent monolayers of Vero E6 cells were infected with SARS-CoV-2 (∼100-200 PFU/well). After 1 h of virus adsorption, the inoculum was removed and replaced with post-infection media containing 1% Avicel with 2-fold serially diluted NAb-containing sample. A and B) At 24 h p.i., cells were fixed with 10 % formalin solution, immunostained with 1 μg/mL of a SARS-CoV cross-reactive NP mAb (1C7), and developed by POD anti-mouse Ab. The neutralizing titer 50 (NT50) was calculated as the highest dilution of the NAb-containing sample that prevents 50 % plaque formation in infected cells, determined by a sigmoidal dose response curve. Dotted line indicates 50 % neutralization. Data were expressed as mean and SD from triplicate wells.