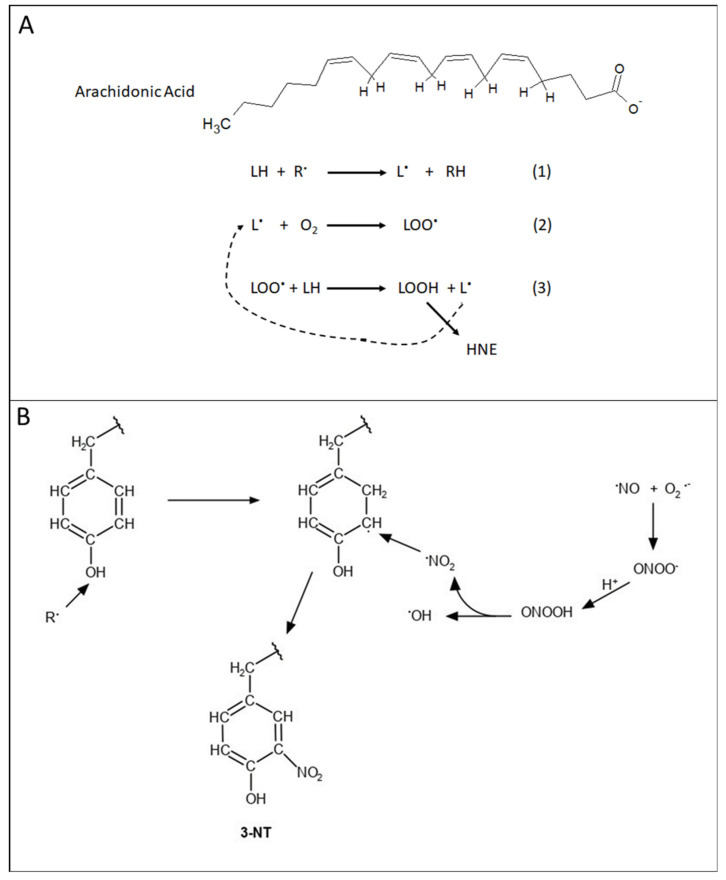
Figure 2.
(A) Mechanism of lipid peroxidation. Arachidonic acid structure showing labile allylic H-atoms that are subject to free radical-mediated abstraction (Rxn 1) leading to carbon-centered free radical on the lipid-resident arachidonic acid carbon backbone. Paramagnetic molecular oxygen, in a radical–radical recombination reaction (Rxn 2), produces the lipid-bound peroxyl free radical. This latter free radical abstracts another labile allylic H-atom (Rxn 3) to form the lipid hydroperoxide and another carbon-centered free radical. Then, this latter radical takes part in Rxn 2 again, i.e., a chain reaction, that will continue as long as there are molecular oxygen and allylic H-atoms present. The lipid hydroperoxide is decomposed into reactive aldehydes, including the highly reactive 4-hydroxynonenal (HNE). See text for additional details. (B). Formation of 3-nitrotyrosine. Nitric oxide (NO), a free radical produced by nitric oxide synthase from arginine, reacts with superoxide free radical anion (see Figure 1) by radical–radical recombination to form peroxynitrite, ONOO−. The actual next reaction shown is more complicated than depicted, but in essence, the protonation of peroxynitrite forms peroxynitrous acid that decomposes into the free radical nitrogen dioxide (NO2) and hydroxyl free radical. Tyrosine, attacked by a free radical R. to remove the H-atom of the phenolic OH group, leaves an unpaired electron on the O-atom that delocalizes to the 3-position of tyrosine with the H-atom removed from the 3-position, reforming the OH functional group on Tyr. NO2 reacts with the delocalized unpaired electron on the 3-position of tyrosine residues in a radical–radical recombination reaction to form 3-nitrotyrosine (3-NT), which is a major marker of nitrosative stress. See the text for further details.