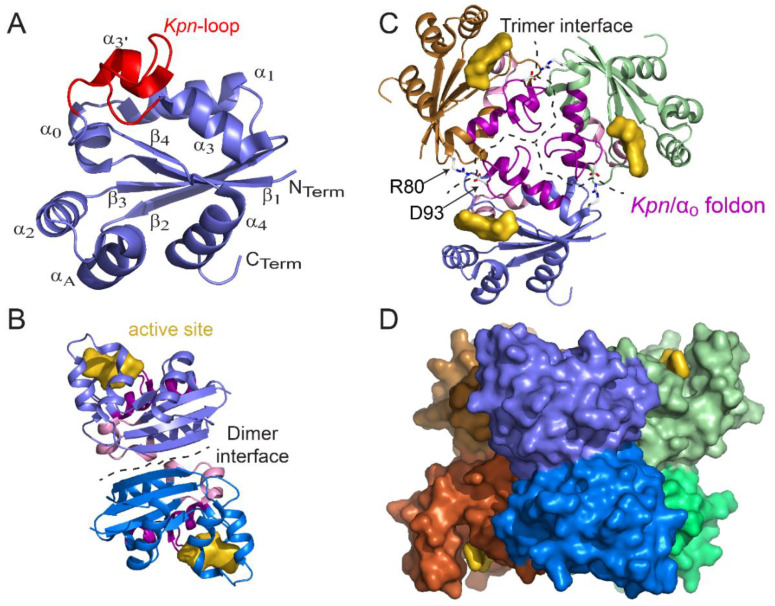
Figure 2.
Structure of monomer, dimer, trimer, and hexamer of NDPK from M. tuberculosis (Figure adapted with permission from [36]). (A) View of the monomer with labeled secondary structural elements. (B) Side view of a dimer showing the dimer interface (residues 17, 19–21, 23, 24, 27, 33–38, and 72) and the active site pocket (K10, Y50, R104, N114, H117, S119, and E128). (C) Top view of a trimer. At the trimer interface (residues 16, 25, 28–31, 79, 80, 83, 84, 87, 88, 93–96, 98–102, and 105–110), the Kpn/α0 foldon (the Kpn-loop and the α0 helix are colored magenta and pink, respectively) and the R80–D93 salt bridge (sticks) are involved in hexamer assembly. The trimer stacks in a “head-to-head” manner and not in a “head-to-tail” manner such that the Kpn/α0 foldon is exposed on either side of the hexamer. (D) Side view of the surface of the six-color hexamer Mt-NDPK. The active site is colored yellow. In panels B and C, the chains are colored as in panel D. All structure figures were drawn using PyMOL molecular graphic system [38].