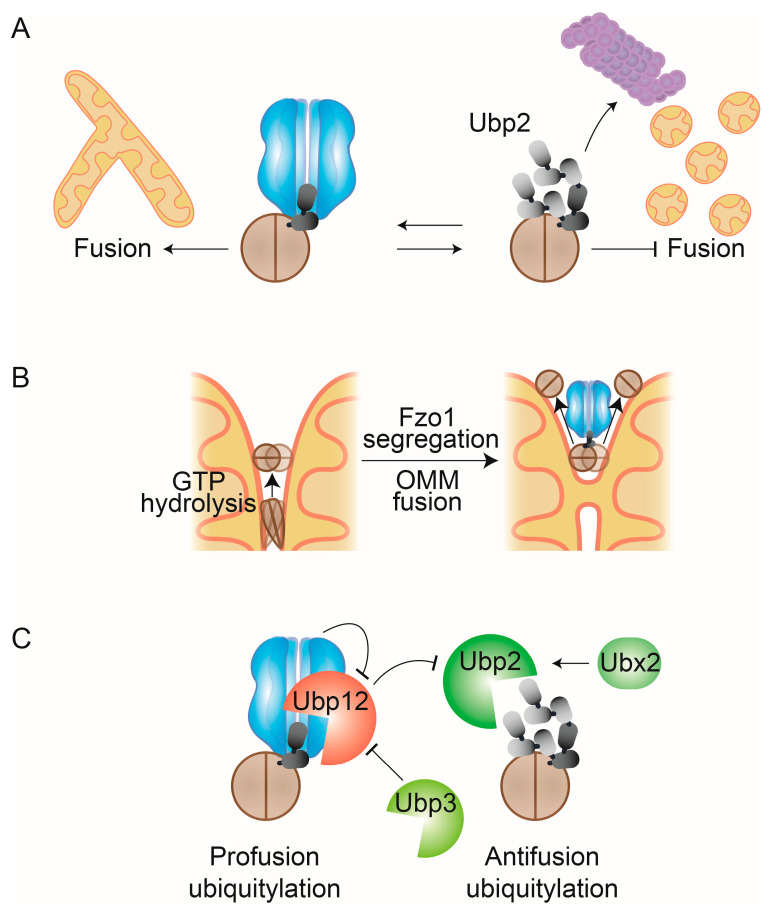
Figure 5.
Regulation of OMM fusion by Cdc48. (A) After GTP hydrolysis, Fzo1 is modified by short ubiquitin chains that are recognized by Cdc48 and that promote mitochondrial fusion. Further ubiquitylation of Fzo1creates a degradation signal that instead targets Fzo1 for degradation by the proteasome, thereby inhibiting fusion. (B) Cdc48 enables Fzo1-dependent membrane merging. OMM merging requires relocalization of mitofusins away from the site of membrane merging, likely mediated by conformational changes of Fzo1, dependent on GTP-hydrolysis. After GTP-hydrolysis Fzo1 can be ubiquitylated and recognized by Cdc48. This leads to complex segregation, allowing OMM fusion and recycling of Fzo1. (C) Cdc48-dependent DUB cascade. Profusion ubiquitylated Fzo1 forms are cleaved by the DUB Ubp12, while antifusion forms, which are built on previously attached regulatory forms, are cleaved by the DUB Ubp2. Cdc48 inhibits Ubp12 activity, while Ubp12 inhibits Ubp2 activity. Thereby, Cdc48 promotes fusion by firstly inhibiting cleavage of profusion ubiquitin forms on Fzo1 and secondly by promoting cleavage of proteolytic ubiquitin forms on Fzo1. Furthermore, Ubp12 is negatively regulated by the DUB Ubp3 while Ubp2 is stabilized by Ubx2.