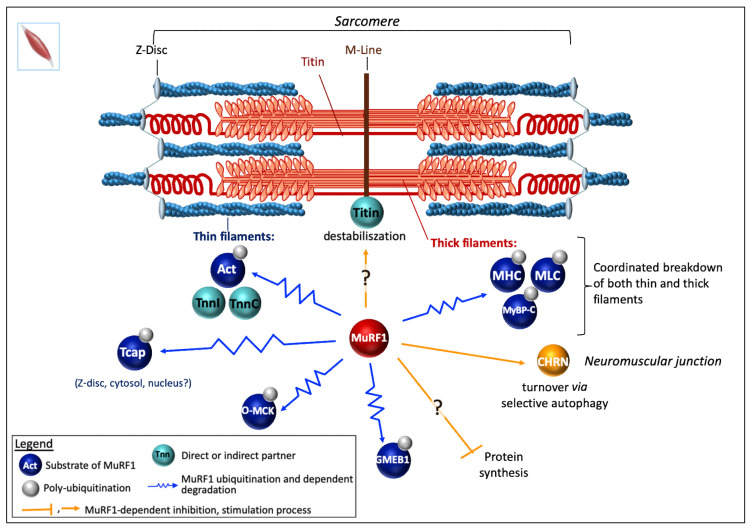
Figure 3.
MuRF1 functions in skeletal muscle. MuRF1 is responsible for the coordinated breakdown of both thick and thin filament occurring during catabolic states in skeletal muscle. Like in cardiac muscle, MuRF1 probably binds to titin at the M-line in some conditions (catabolic conditions, MuRF1 excess, etc.), causing the disruption of titin/M-line interactions and hence a partial disorganization of sarcomere assembly. Upon sarcomere destabilization, MuRF1 would have access to contractile proteins (alpha-actin, troponins I, C, and T, MHC, MLC, MyBP-C), to target them for polyUb and proteasome-dependent degradation. MuRF1 also drives telethonin (Tcap) degradation, but it remains to determine which form of the telethonin is targeted by MuRF1: The free form, the titin-complexed form, or both. The inhibition of protein synthesis by MuRF1 has been reported in skeletal muscles, but more investigations are needed to confirm this role. MuRF1 was reported to target the non-functional oxidized form of muscle creatine kinase (O-MCK). CHRN is targeted to the LC3-II-positive autophagosome for selective autophagy under catabolic condition. This process is regulated and/or mediated partly by MuRF1 and its interacting partners Bif-1/EndoB1 and SQSTM1/p62. Act, alpha-actin; CHRN, cholinergic receptor, nicotinic/nicotinic acetylcholine receptor; GMEB1, glucocorticoid modulatory element binding protein-1; MHC, myosin heavy chain; MLC, myosin light chain; MyBP-C, myosin binding prot-C; O-MCK, oxidized form of muscle creatine kinase; TnnI, TnnC, troponin I and C.