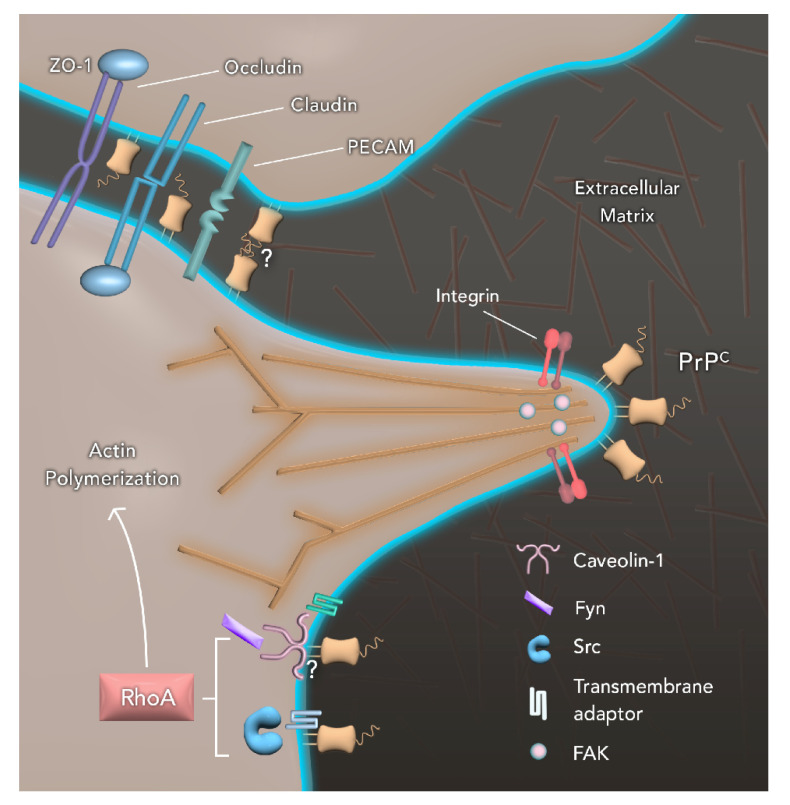
Figure 1.
Cellular prion protein (PrPC) participates in cell motility through interaction with several partners. PrPC can promote the activation of Fyn in a caveolin-1-dependent manner, although the exact mechanism is unknown. Additionally, PrPC can also modulate the activation of Scr. Since PrPC is a glycophosphatidylinositol (GPI)-anchored protein and all the aforementioned proteins are cytosolic, it is postulated the existence of a transmembrane adaptor that acts as an intermediator. Both Fyn and Src are essential for the regulation of Ras homolog family member A (RhoA) activity. In turn, RhoA has a role in the actin polymerization and assembly of F-actin, as well as the formation of cell protrusions such as focal adhesions (FA) with the presence of focal adhesion kinase (FAK), a central regulator of FA. At the tip of FAs is observed an agglomeration of PrPC, as well as integrins and, since PrPC is known to interact with molecules from the extracellular matrix (ECM), it is postulated that both proteins have an important role in adhesion to the extracellular matrix during cell movement. Additionally, PrPC is also known to modulate adhesiveness, being found at the tip of FA and co-localizing with key proteins in cell–cell adhesion domains such as occludin, claudin, PECAM and ZO-1. It is not yet clear if PrPC can interact homophilically with other PrPC molecules from the neighboring cells, although a co-localization is observed.