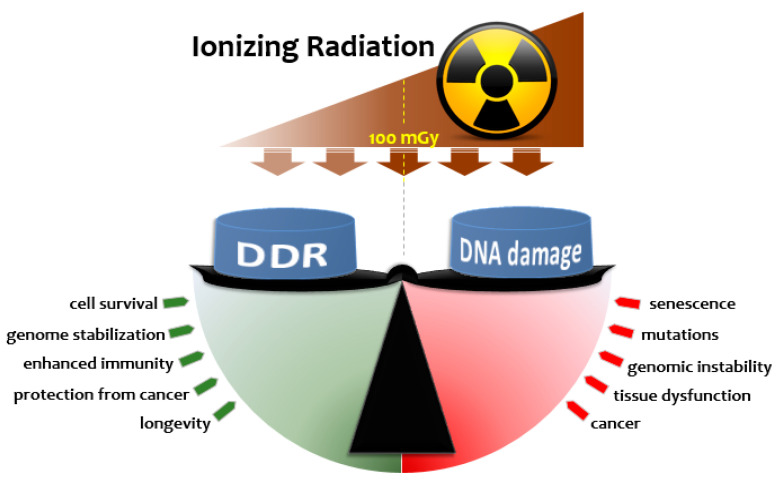
Figure 2.
A dynamic interplay between the amount of DNA damage and DNA damage response (DDR) upon exposure to IR determines the biological outcome in cellular and organismal contexts. Initial DNA lesions caused by exposure to IR are proportional to dose and trigger the DDR; a signaling cascade that senses damage and activates various DNA repair mechanisms, cell cycle arrest, if required, antioxidant defense and other relevant pathways. The magnitude of DDR and downstream branching to more specialized pathways (e.g., survival vs. apoptosis or homologous recombination [HR] vs. non-homologous end joining [NHEJ] DNA repair) may depend on various factors, such as dose, dose rate, radiation type and linear energy transfer, cell type and, microenvironment. Upon exposure to LDR, the DDR triggered is thought to not only repair the low amount of DNA damage caused, but also to render cells resistant to subsequent genotoxic stresses (a radioadaptive response). Such LDR-induced adaptation may last long enough to suppress the rates of mutation, genomic instability, senescence/aging and tumorigenesis caused by either HDR or endogenously generated reactive oxygen species, resulting in radiation hormesis. If, however, the degree of DNA damage produced by IR is high enough—typically above a certain threshold dose that may vary depending on cell type/organism—the capacity of the triggered DDR is insufficient to complete repair. This causes detrimental consequences, such as mutations, genomic instability, neoplastic transformation or tissue dysfunction. The interplay between the DDR and DNA damage is, therefore, dynamic and depends on a multitude of contextually determined factors.