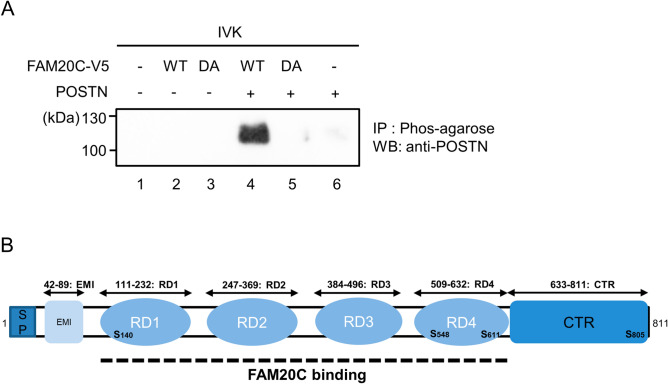
Figure 5.
Periostin is phosphorylated by FAM20C WT, but not D478A kinase inactive form in vitro. (A) In vitro kinase assay was performed by incubating recombinant Periostin and FAM20C WT or kinase inactive D478A, Phos-tag agarose was added and phosphorylated proteins were isolated. Proteins were separated by 4–12% SDS-PAGE and Western blot analysis was performed using anti-Periostin antibody. Only when Periostin was incubated with FAM20C WT, but not D478, Periostin was detected, demonstrating that Periostin was phosphorylated by FAM20C-WT in vitro. (B) Schematic presentation of protein domain structures in mouse Periostin. Mouse Periostin protein structure is illustrated (811 amino acids based on NCBI Reference Sequence; NP_056599.1). Each domain was symbolized such as Signal peptide domain (SP), EMI domain (EMI), Fas I domains/repeated domains (RD1-4), and carboxyl-terminal region domain (CTR). A dotted line indicates FAM20C binding region based on our present study. Potential FAM20C phosphorylation Ser sites (S-X-E) are indicated in bold letters.