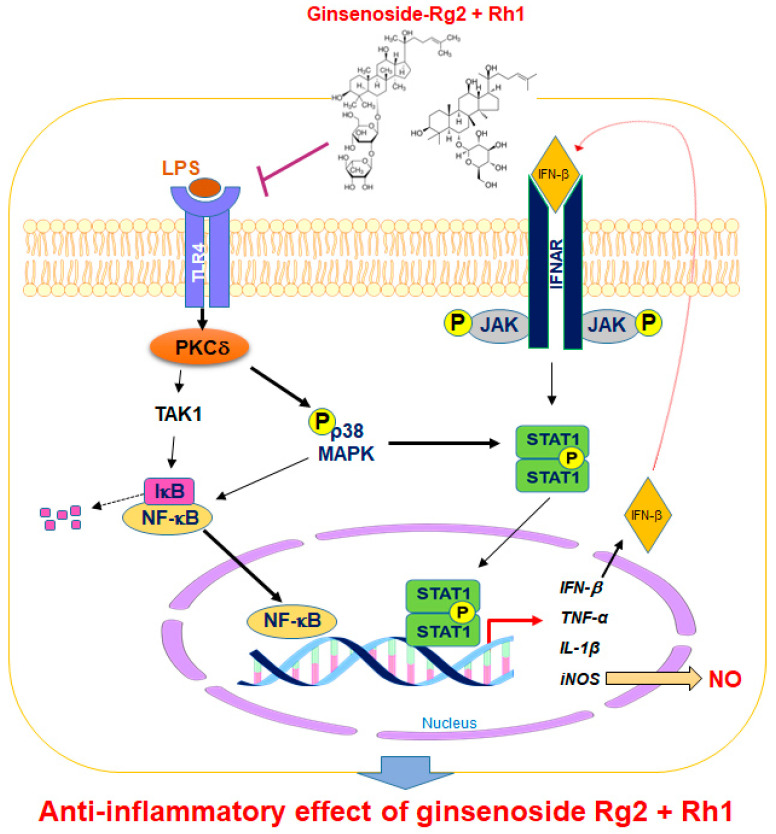
Figure 7.
A schematic diagram showing the signaling pathways involved in anti-inflammatory effects of the combination of ginsenosides Rg2 and Rh1 in macrophages. The combination of ginsenosides Rg2 and Rh1 suppressed inflammation by abolishing the binding of LPS to TLR4, thereby inhibiting the TLR4-mediated signaling pathway. The combined ginsenosides Rg2 and Rh1 synergistically blocked LPS-TLR4 signaling-mediated PKCδ translocation to the plasma membrane, resulting in the decrease in p38-STAT1 activation and NF-κB translocation. Additionally, the activations of p38 and IFN-β are associated with the phosphorylation of STAT1, which facilitates the dimer formation of STAT1. This allows STAT1 enter the nucleus and induce iNOS production. The NF-κB translocation to the nucleus caused the transcription of inflammatory cytokines and mediators such as IFN-β, TNF-α, IL-1β, and iNOS, but ginsenosides Rg2 and Rh1 significantly inhibited these inflammatory markers. LPS, lipopolysaccharide; TLR4, toll-like receptor 4; TAK1, transforming growth factor β-activated kinase 1; PKC-β, protein kinase C delta; NF-κB, nuclear factor-kappa B; IκB, inhibitor of kappa B alpha; MAPK, mitogen-activated protein kinase; JAK, janus kinase; STAT1, signal transducer and activator of transcription 1; IFN-β, interferon-beta; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1 beta TNF-α, tumor necrosis factor-alpha; NO, nitric oxide; P, phosphorylation.