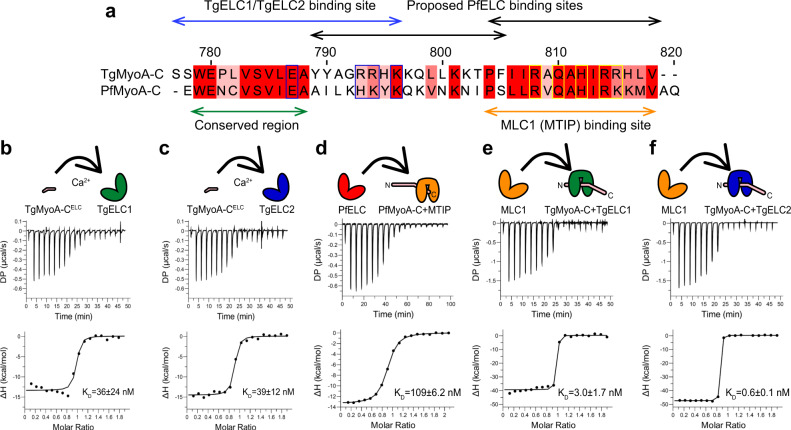
Fig. 3. Assembly of glideosome sub-complexes in T. gondii and P. falciparum.
a Sequence comparison of TgMyoA and PfMyoA C-termini shows a conserved region (green arrow) upstream of the MLC1 (MTIP) binding site. Whereas two binding sites of PfELC at the very C-terminus of PfMyoA were proposed (black arrows)14, our data show that the actual binding site of PfELC encompasses the MyoA conserved region and is similar to the TgELC/TgMyoA binding site (blue arrows). The blue boxed residues indicate residues involved in polar interactions with TgELC1 and TgELC2, while yellow boxed residues form polar interactions with MLC1 (see Fig. 4 and Supplementary Data). b, c Isothermal titration of TgMyoA-CELC with TgELC1 and TgELC2 show that both dimeric complexes form with nanomolar affinity. The upper panel shows the signal recorded directly after each injection of TgELC1 and TgELC2 and represents the thermal power that has to be applied to maintain a constant temperature in the sample cell during recurring injections. In the lower panel, the integrated heats are plotted against the peptide/protein concentration ratio. The thermodynamic binding parameters were obtained by nonlinear regression of the experimental data using a one-site binding model. d Binding isotherm of PfELC titrated to the preformed MTIP/PfMyoA-C complex proves that the conserved hydrophobic region of MyoA is indispensable for ELC binding. e, f Binding isotherms of MLC1 titrated into the pre-complex of TgMyoA-C with TgELC1 and TgELC2. MLC1 binds the pre-complex with high nanomolar affinity. All thermodynamic parameters derived from ITC measurements are summarized in Table 1.