Abstract
Fibroblast growth factor receptors (FGFRs) are tyrosine kinase receptors involved in many biological processes. Deregulated FGFR signaling plays an important role in tumor development and progression in different cancer types. FGFR genomic alterations, including FGFR gene fusions that originate by chromosomal rearrangements, represent a promising therapeutic target. Next-generation-sequencing (NGS) approaches have significantly improved the discovery of FGFR gene fusions and their detection in clinical samples. A variety of FGFR inhibitors have been developed, and several studies are trying to evaluate the efficacy of these agents in molecularly selected patients carrying FGFR genomic alterations. In this review, we describe the most frequent FGFR aberrations in human cancer. We also discuss the different approaches employed for the detection of FGFR fusions and the potential role of these genomic alterations as prognostic/predictive biomarkers.
Keywords: fibroblast growth factor receptors, FGFR fusions, next generation sequencing, cancer, FGFR inhibitors
1. Introduction
Fibroblast growth factor receptors (FGFRs) are highly conserved tyrosine kinase receptors that play an important role in human cancer. Following the binding of growth factors of the fibroblast growth factor (FGF) family, FGFRs dimerize and activate intracellular signaling pathways responsible of cellular proliferation and survival [1]. The FGFR system regulates several crucial developmental processes, including the induction of organogenesis and morphogenesis, and homeostatic processes in adult tissues, such as repair and remodeling [2]. Aberrant activation of FGFRs is observed in different cancer types and plays a role in tumor development and progression. Deregulated FGFR signaling results from different mechanisms. Overexpression of FGFRs might occur either in the presence or absence of genetic alterations (i.e., gene amplification). Mutations (single nucleotide variants, SNVs) of FGFRs have been described in different tumor types. Aberrant activation of FGFRs might also be driven by autocrine and paracrine circuits supported by increased synthesis and release of FGFR ligands [3]. Chromosomal rearrangements leading to FGFR gene fusions have been also found to be involved in the pathogenesis of human cancer.
Gene fusions are hybrid genes that originate from the chromosomal rearrangement of two genes, in the form of translocation, insertion, inversion, and deletion [4]. Fusion events, which involve a variety of partner genes, result in the formation of fusion proteins capable of oncogenic transformation and induction of oncogene addiction. The discovery of targetable fusions and the improvement of techniques used for detecting these alterations allowed the development of specific therapies for the treatment of fusion-driven tumors [5].
The growing therapeutic relevance of FGFR alterations, including fusions, in different cancer types has greatly supported the development of a variety of novel agents along with the improvement of diagnostic tests. In this review, we will focus on the biology of the FGFR system and on the frequency of FGFR aberrations in human cancer. We will also describe the different approaches employed for the detections of fusions and the potential role of these genomic alterations as prognostic/predictive biomarkers.
2. The FGFR/FGF System
The FGFR family comprises four highly conserved tyrosine kinase receptors (RTKs): FGFR1, FGFR2, FGFR3, and FGFR4, consisting of three extracellular immunoglobulin (Ig)-type domains (D1–D3), a single transmembrane domain, and a cytoplasmic tyrosine kinase domain [6]. A unique characteristic of FGFRs is the presence of an acidic, serine-rich sequence, termed the acid box, in the linker region between D1 and D2. The D2–D3 region is necessary for ligand binding and specificity. The D1 domain and the acid box seem to play a role in FGFR autoinhibition [7]. A fifth member of the FGFR family has been discovered, termed fibroblast growth factor receptor-like 1 (FGFRL1/FGFR5), which interacts with heparin and FGF ligands [8]. Like the other members of the FGFR family, FGFR5 consists of three extracellular Ig-like domains and a single transmembrane helix, but it lacks the tyrosine kinase domain, which is replaced by a short intracellular tail with a peculiar histidine-rich motif [9]. The biological function of FGFR5 is unclear. A recent study suggested that it functions as a cell–cell adhesion protein, acting as a tumor suppressor gene [10].
Alternative splicing in the D3 domain of FGFR1, 2, and 3, generates isoforms IIIb and IIIc with different FGF-binding specificity. The IIIb isoforms are predominantly expressed in epithelial tissues, whereas IIIc isoforms are expressed in mesenchymal tissues. Alternative splicing and switching from epithelial to mesenchymal isoforms are involved in the epithelial-to-mesenchymal transition and in tumor progression [11]. In this regard, genomic rearrangements leading to the generation of fusion proteins might also alter the splicing of FGFR isoforms. However, no data on the involvement of this phenomenon in the growth of cancer addicted to FGFR fusions are available. Soluble splice variants of FGFR4 have been recently described, although further studies are required to better define the biological functions of these isoforms [12,13].
The FGF family of proteins is composed of 18 ligands (FGF1–FGF10 and FGF16–FGF23). Members of five of the six subfamilies act as paracrine factors, whereas members of the FGF19 subfamily (FGF19, FGF21, and FGF23) work in an endocrine fashion [7]. Four FGF homologous factors (previously indicated as FGF11–FGF14) fail to activate any FGFRs and are not considered members of the FGF family [14], whereas FGF15 is the mouse orthologue of FGF19. FGF ligands interact with heparan sulfate proteoglycans that are present both at the cell surface and in the pericellular and extracellular matrix. Heparan sulfate proteoglycans are obligatory cofactors of paracrine FGFs for FGFR activation, whereas endocrine FGFs preferentially require Klotho proteins as co-receptors to initiate FGFR signaling [15].
Ligand binding to the receptor induces FGFR dimerization and the subsequent phosphorylation of the tyrosine kinase domain. Activation of the receptor promotes the phosphorylation of intracellular substrates, including FGFR substrate 2α (FRS2α) and phospholipase Cγ1 (PLCγ1). FRS2α activates RAS/MEK/ERK and PI3K/AKT signaling pathways that regulate cell proliferation and survival, whereas PLCγ1 stimulates cell motility through the activation of protein kinase C (PKC) and calcium-dependent proteins [2]. Other pathways are activated by FGFRs, including JAK/STAT, p38MAPK, Jun N-terminal kinase, and RSK2 [16]. Different negative regulators, including Sprouty proteins and MAPK phosphatase 3 attenuate FGFR signaling [6].
3. Genetic Alterations of FGFRs in Human Cancers
Deregulated FGFR signaling is observed in various tumor types. A recent study that analyzed the FGFR genomic alterations in 4853 tumor samples by next-generation sequencing (NGS), described the presence of FGFR alterations in 7.1% of cases [17]. Genetic aberrations of FGFR1 are more frequently observed in human cancers (2.86%), followed by alterations in FGFR3 (2.21%), FGFR2 (1.77%), and FGFR4 (1.54%).
3.1. FGFR Amplifications and Mutations
Gene amplifications are the most frequent FGFR alterations reported in human cancers accounting for 66% of all FGFR aberrations [17]. Gene amplification often leads to the overexpression of FGFR proteins, resulting in the aberrant activation of the receptors and an increased downstream signaling [1]. FGFR1 is the most commonly amplified gene (2.25%) [18]. FGFR1 amplification is frequently observed in breast, lung, and colon cancer [18]. FGFR2 amplification is less frequent (0.34%) and has been described in some cancer types, including breast, gastric, and esophageal carcinoma [18]. FGFR3 gene amplification (0.31%) has been observed in breast carcinoma, bladder carcinoma, glioblastoma multiforme, pancreatic cancer, and lung adenocarcinoma. FGFR4 amplification is rare (0.16%).
FGFR mutations are less frequent than FGFR amplifications, representing 26% of the aberrations detected in FGFR-altered tumors [17]. FGFR mutations can affect the extracellular or the transmembrane or the kinase domains of FGFRs and result in a deregulated FGFR signaling through various mechanisms, including the increased kinase activation, the reduced degradation of the receptor, or the abnormal receptor dimerization [1,16]. Mutations in FGFR1 have been observed in 1.12% of cases, with a prevalence in lung, colon, breast, endometrial adenocarcinoma, and glioblastoma multiforme. The most frequent FGFR1 activating mutation is the N546K (0.12%) [18] in the kinase domain of the receptor that alters the tyrosine auto-phosphorylation with an increased kinase activation [19]. Mutations in FGFR2 and FGFR3 are more frequent (1.36% and 1.83%, respectively) [18]. The most common FGFR2 activating mutations are the S252W mutation in the extracellular domain (0.17%), the N549K mutation in the tyrosine kinase domain (0.06%), and the C382R mutation affecting the transmembrane domain of the receptor (0.06%) [18]. The most frequent FGFR3 activating mutation is the S249C missense mutation that resides in the extracellular domain of the receptor (0.54%) [18]. This mutation induces ligand-independent dimerization and constitutive phosphorylation of the receptor [20]. The FGFR3 S249C mutation is relatively frequent in bladder cancer (66.6%) [21]. FGFR4-activating mutations are rare and are detected in some pediatric tumors, such as rhabdomyosarcoma [22]. A novel oncogenic mutation of FGFR4 (G636C) has been recently discovered in gastric cancer [23].
3.2. FGFR Family Gene Fusions
FGFR fusions have been described in several tumor types, although the incidence is low (8%) [17]. FGFR fusions can be classified into type I or type II fusions. In type I fusions, where the FGFR is the 3′ fusion partner, the extracellular and the transmembrane domains are excluded from the fusion protein, which includes only the FGFR kinase domain linked to the 5′ protein partner. In type II fusions, with the FGFR as the 5′ fusion gene, the breakpoint usually occurs in exons 17, 18, or 19, and the extracellular, the transmembrane, and the kinase domain remain intact [16]. In both types of fusion proteins, the diverse FGFR fusion partners contribute with specific domains that favor the dimerization, including the coiled-coil, the SPFH, the sterile alpha motif (SAM), the LIS1-homologous (LisH), the IMD, and the caspase domains [24]. Such ligand-independent increased dimerization provides oncogenic potential to the FGFR fusion protein. Fusion genes between FGFR1–2–3 and multiple partners have been identified in several tumor types (Table 1). It is rare to find FGFR fusions together with FGFR mutations, suggesting that the presence of unique alterations is sufficient to drive cancer progression.
Table 1.
Most frequent fibroblast growth factor receptor (FGFR) fusions in solid tumors.
| Gene | 5′-Gene | 3′-Gene | Tumor Type | No. of Cases Reported (Ref.) |
|---|---|---|---|---|
| FGFR1 | FGFR1 | HOOK3 | GIST | 1/186 [25] |
| FGFR1 | TACC1 | GIST | 1/186 [25] | |
| Glioma | 1/795 [26] | |||
| Glioblastoma | 1/97 [27] | |||
| FGFR1 | ZNF703 | Breast cancer | 1/24 [28] | |
| FGFR1 | NTM | Bladder urothelial carcinoma | 1/295 [29] | |
| BAG4 | FGFR1 | Non-small cell lung cancer | 2/1328 [30]; 1/26,054 [31] | |
| FGFR2 | FGFR2 | AHCYL | Cholangiocarcinoma | 7/102 [32] |
| FGFR2 | BICC1 | Cholangiocarcinoma | 2/102 [32]; 6/195 [34]; 8/377 [35]; 40/107 [37]; | |
| Colorectal cancer | 1/149 [32]; | |||
| Hepatocarcinoma | 1/96 [32] | |||
| FGFR2 | PPHLN1 | Cholangiocarcinoma | 16/107 [37] | |
| FGFR2 | TACC3 | Cholangiocarcinoma | 1/6 [36] | |
| FGFR2 | CCDC6 | Cholangiocarcinoma | 3/377 [35] | |
| FGFR2 | KIAA1598 | Non-small cell lung cancer | 2/26054 [31] | |
| FGFR3 | FGFR3 | TACC3 | Glioblastoma | 2/97 [27] |
| Glioma | 20/795 [26] | |||
| Non-small cell lung cancer | 15/1328 [30]; 37/26,054 [31] | |||
| Bladder cancer | 3/2375 [38] | |||
| Head and neck squamous cancer | 2/2375 [38] | |||
| Lung squamous cell carcinoma | 4/2375 [38] | |||
| FGFR3 | BAIAP2L1 | Bladder cancer | 1/2375 [38]; 2/46 [44] | |
| Lung cancer | 2/83 [44] |
GIST, gastrointestinal stromal tumor.
FGFR1 fusions are rare in solid tumors. A FGFR1–HOOK3 gene fusion has been observed in gastrointestinal stromal tumor (GIST) [25]. FGFR1–TACC1 was detected in GIST, in grade II IDH wild-type glioma, and in glioblastoma [25,26,27], whereas FGFR1–ZNF703 was detected in breast cancer [28]. These fusions involve the N-terminus of the FGFR1 protein and the coiled coil of the fusion partners to induce activation of the receptor and downstream signaling. The FGFR1–NTM fusion, whose functional effect is unknown, was detected in bladder urothelial carcinoma [17,29]. BAG4–FGFR1 was identified in non-small cell lung cancer (NSCLC) [30,31].
FGFR2 fusions are the most frequent FGFR fusions [17]. As compared with the other member of the FGFR family, FGFR2 had several reported partners and FGFR2 fusions are particularly common in cholangiocarcinoma. In this regard, FGFR2–AHCYL, FGFR2–BICC1, FGFR2–PPHLN1, and FGFR2–TACC3 fusions have been frequently described in patients with intrahepatic cholangiocarcinoma, although over 100 different FGFR2 partners have been reported in this disease [32,33,34,35,36,37,38]. These fusions activate the canonical FGFR signaling and possess oncogenic activity [37,39]. The FGFR2–CCDC6 fusion has been demonstrated to induce cancer cell proliferation and tumorigenesis in vivo [38,40]. Several other partners involved in FGFR2 fusion genes, whose biological activity has not been fully characterized, have been described in cholangiocarcinoma, including KIAA1217, KIAA1598, DDX21, LAMC1, NRAP, NOL4, PHC1, RABGAP1L, RASAL2, ROCK1, TFEC, AFF4, CELF2, DCTN2, DNAJC12, DZIP1, FOXP1, INA, KCTD1,LGSN, LPXN, MYPN, PRKN, PCM1, RNF41, SH3GLB1, STK3, SORBS1, TBC1D1, and UBQLN1 [33,34,35].
FGFR2–BICC1 has been also identified in colorectal cancer and hepatocarcinoma, although with low frequency [32]. Two FGFR2–KIAA1598 fusions and other FGFR2 fusions with novel partners (CIT, ERC1, LZTFL1, POC1B, SORBS1, TP73, TXLNA) have been recently identified in a large cohort (n = 26054) of lung cancer patients [31].
FGFR3 fusions are more commonly observed in glioblastoma, bladder, and lung cancer [18]. The majority of FGFR3 fusions are with transforming acidic coiled-coil 3 (TACC3) and result from the in-frame fusion of the FGFR3 N-terminus with the TACC3 C-terminus [27]. TACC3 protein has a coiled-coil domain at the C terminus and is involved in mitotic spindle assembly and stability [41]. FGFR3–TACC3 fusions have been described in different tumor types, including glioma, lung cancer, bladder cancer, head and neck squamous cancer, lung squamous cell carcinoma, and cervical cancer [26,27,30,31,38,42,43]. The FGFR3–TACC3 fusion protein induces a constitutive activation of the tyrosine kinase domain with the consequent activation of MEK/ERK and STAT1 signaling, but not PLCγ1, as the tyrosine residue in exon 19, responsible for the interaction with PLCγ1, is lost [38,42]. The FGFR3–TACC3 protein also induces mitotic and chromosomal segregation defects and generates aneuploidy [27]. The presence of the FGFR3–TACC3 fusion increased the proliferation of cancer cell lines [27,38] and induced tumorigenesis in mice [27].
Among other FGFR3 fusions, FGFR3–BAIAP2L1 has been described in bladder and lung cancer [42,44]. FGFR3–BAIAP2L1 fusion promotes the constitutive activation of FGFR3 signaling with a potent oncogenic activity [42,44]. Other fusion partners of FGFR3 include AES, ELAVL3, JAKMIP1, TNIP2, and WHSC1 [5,17].
Recently, FGFR4 fusions (ANO3–FGFR4, NSD1–FGFR4) have been identified in NSCLC patients [31].
4. Approaches to Detect FGFR Fusions in Clinical Diagnostics
Since the discovery of the first chromosomal rearrangements in hematologic malignancies using chromosomal-banding techniques, technological advancements have enabled the detection of a wide number of gene fusions in many tumor types [4,5,45,46]. The development of fluorescence in situ hybridization (FISH) technique in combination with cytogenetics allowed the simultaneous visualization of different chromosome structures in different colors, significantly improving the localization of chromosomal breakpoints. This approach employs fluorescently labeled DNA probes that bind to specific complementary target sequences. Detection of the signals is performed by fluorescence microscopy [47]. In particular, the break-apart FISH assay allows the identification of gene translocations using probes specific for loci that are physically close in the wild-type configuration. The wild-type signal pattern shows two pairs of closely approximated or fused signals, whereas the two colors split apart when a translocation occur [48].
As compared to standard cytogenetics, FISH analyses do not require living cells; can be easily performed on clinical formalin-fixed, paraffin-embedded (FFPE) samples; and are a technique with a relatively fast turnaround time. However, the resolution is low, and complex rearrangements are not usually easily detectable. Intrachromosomal rearrangements, which account for about 50% of FGFR2 fusions in intrahepatic cholangiocarcinoma, can also lead to false-negative results of FISH analysis. In addition, the analysis is mainly restricted to the detection of DNA. FISH analysis, using break-apart probes, has been frequently used to detect FGFR fusions in clinical samples [36,44]. Recently, a novel RNA-FISH assay allowed the detection of FGFR3–TACC3 fusions in bladder cancer [49].
Immunohistochemistry can also detect fusions when rearrangements lead to overexpression of the kinase. Immunohistochemistry is inexpensive and provides information about specific fusions by protein localization, but this approach has a very low sensitivity in identifying rare fusions. So far, no immunohistochemistry method has been proven to have sufficient sensitivity and specificity to detect FGFR fusions [30].
The introduction of NGS technologies, able to identify different types of genomic alterations, including fusions either at DNA or RNA level in a single experiment, allowed the discovery of about the 90% of about 10,000 known gene fusions [4]. Among the NGS analytical strategies, whole-genome sequencing (WGS) has the advantage of identifying a large number of rearrangements and characterize breakpoints, including those in non-coding regions, and it is particularly useful for the discovery of novel fusions. However, this approach is very expensive and time consuming, due to the large quantity of data generated and computational analyses. Whole-exome sequencing (WES) is less suitable than WGS, as it can detect few rearrangements with breakpoints in or near exons [50]. Instead, whole-transcriptome sequencing has been used for the discovery of several gene fusions in different cancer types [32,51]. As compared with WGS, RNA-based testing is more sensitive, efficient, and functionally definitive considering that, although many rearrangements might be present in the genome of tumor samples, only few produce transcripts. A next-generation transcriptome approach was indeed used for the discovery of the first FGFR family gene fusion, FGFR3–TACC3 in glioblastoma multiforme [27]. However, whole-transcriptome analysis is quite expensive, requires personnel with expertise in bioinformatics for data analysis and interpretation, and is not applicable for routine clinical-grade testing.
Targeted sequencing approaches that allow the isolation and sequencing of subsets of genes or regions of the entire genome can detect fusions in a more focused manner. This strategy is a sensitive approach in detecting fusions, as the sequencing coverage is higher than that of WGS. In addition, targeted sequencing is a suitable approach for detecting fusions in clinical diagnostics, as it can investigate DNA and/or RNA and does not require extensive validation of the method, being available several CE-IVD panels (Table 2). DNA-based methods have the advantage that DNA is more stable than RNA, but the detection of novel fusions might be limited, especially when large intronic regions are involved. RNA-based methods are able to distinguish in-frame, transcribed gene fusions versus out-of-frame fusions and avoid difficulties of sequencing large intronic regions. The main weaknesses of RNA-based methods are that the sensitivity depends on the fusion expression level and that RNA is less stable than DNA, especially when FFPE samples are used.
Table 2.
Commercially available targeted sequencing kits for fusion detection.
| Technology | Kit | Sample | Nucleic Acid | Input | No. of Genes | No. of Fusion Genes |
|---|---|---|---|---|---|---|
| Hybrid Capture-based | FoundationOneCDx (Foundation Medicine) | FFPE | DNA | Moderate (≥50 ng FFPE RNA) | 324 | 36, including FGFR1–3 |
| TruSight Tumor 170 (Illumina) | FFPE | DNA/RNA | Moderate (≥40 ng FFPE DNA/RNA) | 170 | 55, including FGFR1–4 | |
| TruSight Oncology 500 (Illumina) |
FFPE | DNA/RNA | Moderate (≥40 ng FFPE DNA/RNA) | 523 | 55, including FGFR1–4 | |
| Amplicon-based | Oncomine comprehensive assay (Thermofisher) | FFPE | DNA/RNA | Low (≥10 ng FFPE DNA/RNA) | 161 | 51, including FGFR1–3 |
| Oncomine Focus Assay (Thermofisher) | FFPE | DNA/RNA | Low (≥10 ng FFPE DNA/RNA) | 52 | 23, including FGFR1–3 | |
| Anchored multiplex PCR-based | FusionPlex Oncology Research(ArcherDX) | Fresh, frozen, and FFPE | RNA | Moderate (≥50 ng § FFPE RNA) | 75 | 75¥, including FGFR1–3 |
| FusionPlex Solid Tumor (ArcherDX) | Fresh, frozen, and FFPE | RNA | Moderate (≥50 ng § FFPE RNA) | 53 | 53 ¥, including FGFR1–3 | |
| FusionPlex Comprehensive Thyroid and Lung (CTL) (ArcherDX) | Fresh, frozen, and FFPE | RNA | Moderate (≥50 ng § FFPE RNA) | 36 | 16 ¥, including FGFR1–3 | |
| FusionPlex Lung (ArcherDX) | Fresh, frozen, and FFPE | RNA | Moderate (≥50 ng § FFPE RNA) | 14 | 13 ¥, including FGFR1–3 |
Abbreviations: FFPE formalin-fixed, paraffin-embedded; § recommended input in the absence of PreSeq screening; ¥ fusion, splicing, or exon-skipping.
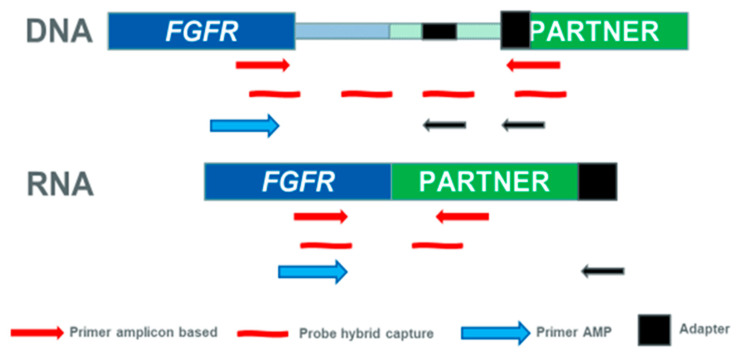
Different approaches are currently available for detecting fusions, i.e., hybrid capture-based methods, amplicon-based approaches, and anchored multiplex PCR (Table 2). Hybridization-capture methods use sequence-specific probes complementary to a specific region of interest that are longer than PCR primers, making it possible to sequence target regions and regions flanking the target. As compared to amplicon-based approaches, hybridization-based strategies are less likely to miss variants, although the detection of fusions might be challenging if introns are long (Figure 1). Amplicon-based enrichment methods use primers specific to known fusion partners and allow the detection of fusions starting from a very low input (≥10 ng) of RNA. This approach can be suitable also for degraded RNA commonly derived from FFPE clinical samples. Other advantages include a simplified workflow, a reduced complexity of data analysis, and a short time for test execution. However, amplicon-based methods limit the discovery of novel fusions, without a prior knowledge of potential partners. A number of FGFR fusions have been detected in clinical samples using these approaches [31,33].
Figure 1.
DNA- and RNA-targeted sequencing approaches for detecting FGFR fusions. Hybrid capture methods use sequence specific probes complementary to a specific region of interest that are longer than PCR primers, whereas amplicon-based enrichment methods use primers specific to known fusion partners. The anchored multiplex PCR approach uses gene-specific primers and universal reverse primers that permit the amplification of both known and unknown genomic regions of interest. When DNA is used as starting material, hybrid capture probes can be designed to capture both exons and introns. RNA-based methods detect only functional transcripts, avoiding the difficulties of sequencing large intronic regions.
A recent strategy used for target enrichment of RNA libraries for NGS is the anchored multiplex PCR, for example, using the Archer FusionPlex chemistry that uses gene-specific primers anchored to an exon–intron boundary and universal reverse primers that permit the amplification of both known and unknown genomic regions of interest (Table 2 and Figure 1). The main advantage of this technology is the enrichment of a target region with knowledge of only one of its ends [52]. In particular, this method allows the detection of any fusion partner, even if only one of the fusion partners is known, and gives information on the imbalance between 5′ expression and 3′ expression, a phenomenon frequently observed in samples positive for fusions involving a driver gene. Anchored multiplex PCR has been recently used to identify various FGFR2 fusions in cholangiocarcinoma clinical samples [53].
5. Prognostic Significance of FGFR Fusions
The improvement of diagnostic strategies for detection of FGFR alterations allowed the identification of a number of FGFR fusions that might potentially predict the outcome of cancer patients. The prognostic role of FGFR fusions has mainly investigated in biliary tract cancer. In this regard, a study evaluated the presence of FGFR2 translocations in 152 cholangiocarcinoma and 4 intraductal papillary neoplasms of the bile duct by FISH [54]. Thirteen specimens were positive for FGFR2 translocations. The median cancer-specific survival interval for patients carrying FGFR2 translocations was significantly longer (123 months) than that for patients without FGFR2 translocations (37 months, P = 0.039) [54]. In a study in which 377 patients with biliary tract cancer were enrolled, 95 FGFR genetic alterations, including 63 FGFR2 fusions, were detected. Patients with FGFR alterations experienced significantly longer overall survival (OS) than patients without FGFR aberrations (37 vs. 20 months; P <0.001) [35]. In a recent study in patients with fluke associated-intrahepatic cholangiocarcinoma, the presence of rare FGFR2 fusions indicated a trend toward better OS compared with that of fusion-negative tumors, although the difference was not statistically significant [53]. The presence of FGFR genomic alterations, including FGFR2 fusions genes identified by NGS in 55 patients with intrahepatic cholangiocarcinoma, has been associated with an indolent disease course and prolonged survival [55]. However, in this study, FGFR2 fusions have been reported only in three patients. Interestingly, one patient with an FGFR2–NOL4 fusion and a co-existing BAP1 mutation had a rapidly progressive course [55]. In the study of Arai et al. in which seven FGFR2–AHCYL1-positive and two FGFR2–BICC1-positive intrahepatic cholangiocarcinomas were identified, no significant differences in term of prognosis between fusion-positive and -negative patients were observed [32]. In this study, KRAS and BRAF mutations were mutually exclusive with FGFR2 fusions.
The prognostic significance of FGFR1–3 fusions was explored in NSCLC. In a study in which 1328 NSCLC patients were enrolled, 17 had FGFR fusions (2 BAG4–FGFR1 and 15 FGFR3–TACC3) [30]. No significant differences in relapse-free survival (RFS) or OS were observed between patients with FGFR-fusion-positive and FGFR-fusion-negative tumors [30].
6. FGFR Fusions as Therapeutic Target for Solid Tumors
Even though FGFR fusions are rare in human cancers, the field of therapies targeting these molecular alterations has exponentially progressed, thanks to development of a number of novel compounds, including non-selective and selective tyrosine kinase inhibitors (TKIs).
The first anti-FGFR agents were multi-kinase non-selective inhibitors (e.g., dovitinib, lenvatinib, lucitanib, nintedanib, derazantinib, and ponatinib) that, in addition to inhibit FGFRs, are active against different tyrosine kinases, including VEGFRs, RET, KIT, and PDGFRs, due to the similarity of the intracellular tyrosine kinase domains (Table 3). However, these compounds lack specificity and potency for treatment of FGFR-driven tumors, with an increased risk of adverse events at the doses required for FGFR inhibition. In this regard, the occurrence of several adverse events, including cardiovascular effects, have been reported [56,57,58]. Different clinical trials of non-selective TKIs are ongoing in patients with FGFR alterations (Table 3). Few studies reported some activity of these agents in FGFR-driven tumors. In this regard, in a study of dovitinib in 13 patients with Bacillus Calmette–Guerin (BCG)-refractory urothelial carcinoma and FGFR3 alterations, three patients had FGFR3 mutations [58]. The response rate (RR) was 8% with only one complete response (CR) in a patient carrying the FGFR3 S249C mutation. All patients experienced grade 3–4 toxicity [58]. In a clinical study in FGFR2-mutant or wild-type endometrial cancer patients treated with dovitinib, the RR in the FGFR2 mutant group was 5% (11% for all patients); only 1/22 FGFR2 mutant patients achieved a partial response (PR) [57]. Treatment with derazantinib produced an overall RR (ORR) of 20.7%, a disease control rate (DCR) of 82.8%, and a median progression-free survival (PFS) of 5.7 months in patients with advanced, unresectable intrahepatic cholangiocarcinoma and FGFR2 fusions who progressed after chemotherapy [59]. In a phase I/II trial of lucitanib, the clinical activity of the drug was evaluated in an expansion cohort of 23 patients with FGFR-aberrant tumors. The RR was 30.4% with a PFS of 32.1 weeks [60].
Table 3.
Clinical trials of non-selective FGFR inhibitors in patients with solid tumors and FGFR genetic alterations.
| Compound | Target | Eligibility on the Basis of FGFR Alterations | Tumor Type | Phase | ClinicalTrial Identifier |
|---|---|---|---|---|---|
| Dovitinib | FGFR1–2–3; VEGFR1–2–3; PDGFRβ | FGFR3 mutation/over-expression | BCG refractory urothelial carcinoma | II | NCT01732107 |
| FGFR mutation/ amplification/ translocation | Solid and hematologic tumors | II | NCT01831726 | ||
| FGFR2 amplification | Metastatic or unresectable gastric cancer | II | NCT01719549 | ||
| FGFR2 mutation or FGFR2 wild type | Advanced and/or metastatic endometrial cancer | II | NCT01379534 | ||
| Lucitanib | FGFR1–2–3; VEGFR 1–2–3; PDGFRα-β; CSF1R | FGFR 1–3 gene fusion/activating mutation | Advanced/metastatic lung cancer | II | NCT02109016 |
| FGFR aberrations | Advanced cancers | II | NCT02747797 | ||
| FGFR1 amplification or FGFR1 wild type | Estrogen receptor-positive metastatic breast cancer | II | NCT02053636 | ||
| Nintedanib | FGFR1–2–3; VEGFR 1–2–3; PDGFRα-β | FGFR 1–3 alterations | Advanced non-small cell lung cancer | II | NCT02299141 |
| FGFR3 mutation/ overexpression or FGFR3 wild type | Advanced urothelial carcinoma | II | NCT02278978 | ||
| Ponatinib | FGFR1, VEGFR2; BCR–ABL, SRC; KIT; PDGFRα | FGFR mutation/ fusion/amplification | Advanced cancers | II | NCT02272998 |
| FGFR2 fusion | Advanced biliary cancer | II | NCT02265341 | ||
| Derazantinib | FGFR1–3, CSF1R, RET; KIT; PDGFRβ | FGFR aberrations | Advanced urothelial cancer | I/II | NCT04045613 |
| FGFR genetic alterations | Advanced solid tumors | I/II | NCT01752920 | ||
| FGFR2 fusion/ mutation/amplification | Inoperable or advanced intrahepatic cholangiocarcinoma | II | NCT03230318 |
BCG, Bacillus Calmette–Guerin.
More recently, inhibitors that reversibly or irreversibly bind to the adenosine triphosphate (ATP) pocket of FGFRs and selectively inhibit the activity of the receptors have been developed.
Only two selective FGFR-TKIs have been approved up to now by the Food and Drug Administration (FDA) for the treatment of FGFR-driven cancer. In particular, erdafitinib has been approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 genetic alterations, including R248C, S249C, G370C, and Y373C mutations and FGFR3–TACC3 fusions, on the basis of the BLC2001 trial [61]. In this phase 2 study that enrolled 99 patients with advanced urothelial carcinoma carrying FGFR3 or FGFR2/3 genomic alterations, an RR of 40%, with 3% of patients obtaining a CR, was observed. In the subgroup of 25 patients with FGFR fusions, the RR was 16% [61]. Pemigatinib was granted FDA-accelerated approval in April 2020 for the treatment of cholangiocarcinoma patients with FGFR2 fusions or rearrangements. The efficacy of the drug was evaluated in the FIGHT-202 study in 107 patients with cholangiocarcinoma and FGFR2 gene fusions. The ORR was 35.5%, including 3 CRs. No CRs or PRs were observed in patients with other FGF/FGFR alterations or no FGF/FGFR alterations [62].
A number of different reversible competitive inhibitors directed against multiple FGFRs (e.g., erdafitinib, pemigatinib, infigratinib, rogaratinib, AZD4547, Debio1347) are in clinical development in patients with hematologic and solid tumors who carry FGFR alterations (Table 4). FGFR4 selective agents (fisogatinib, H3B-6527, and FGF401) are also under investigation.
Table 4.
Clinical trials with selective FGFR inhibitors.
| Drug | Target | Tumor Type | Phase | Status | Clinical Trial Identifier |
|---|---|---|---|---|---|
| Reversible FGFR inhibitors | |||||
| Erdafitinib (JNJ-42756493) | FGFR1–4 | FGFRaberrant advanced refractory solid tumors, lymphomas, or multiple myeloma | II | recruiting | NCT02465060 |
| FGFR-aberrant urothelial cancer | II | active | NCT02365597 | ||
| FGFR-aberrant advanced squamous non-small-cell lung carcinoma | II | recruiting | NCT03827850 | ||
| FGFR-aberrant urothelial cancer | III | recruiting | NCT03390504 | ||
| ER+/HER2-/FGFR-amplified metastatic breast cancer | I | recruiting | NCT03238196 | ||
| FGFR-aberrant advanced non-small-cell lung cancer, urothelial cancer, esophageal cancer, or cholangiocarcinoma | II | recruiting | NCT02699606 | ||
| Advanced solid tumor with FGFR mutation or gene fusion | II | recruiting | NCT04083976 | ||
| Pemigatinib (INCB054828) | FGFR1–3 | FGFR-aberrant advanced solid malignancies | I/II | recruiting | NCT02393248 |
| FGFR3 mutant or rearranged metastatic or unresectable urothelial carcinoma | II | recruiting | NCT04003610 | ||
| FGFR2 rearranged unresectable or metastatic cholangiocarcinoma | III | recruiting | NCT03656536 | ||
| FGFR-aberrant unresectable advanced, relapsed, or metastatic solid tumors | I | not yet recruiting | NCT04258527 | ||
| FGFR-aberrant metastatic or unresectable colorectal cancer | II | not yet recruiting | NCT04096417 | ||
| FGFR-aberrant metastatic or surgically unresectable urothelial carcinoma | II | recruiting | NCT02872714 | ||
| Locally advanced/metastatic or surgically unresectable solid tumor malignancies with activating FGFR mutations or translocations | II | recruiting | NCT03822117 | ||
| High-risk patients with urothelial carcinoma with activating FGFR mutations or translocations | II | not yet recruiting | NCT04294277 | ||
| Infigratinib (BGJ398) | FGFR1–3 | Advanced or metastatic cholangiocarcinoma with FGFR2 gene fusions or translocations or other FGFR genetic alterations | II | recruiting | NCT02150967 |
| Invasive urothelial carcinoma and FGFR3 genetic alterations | III | recruiting | NCT04197986 | ||
| Advanced or metastatic solid tumors with FGFR1–3 gene fusions or other FGFR genetic alterations | II | Recruiting | NCT04233567 | ||
| Unresectable locally advanced or metastatic cholangiocarcinoma with FGFR2 gene fusions/translocations | III | recruiting | NCT03773302 | ||
| Recurrent high-grade glioma with FGFR1 K656E or FGFR3 K650E mutation or FGFR3–TACC3 translocation | I | recruiting | NCT04424966 | ||
| Rogaratinib (BAY1163877) | FGFR1–4 | Recurrent or metastatic squamous cell carcinoma of the head and neck with FGFR1/2/3 mRNA overexpression | II | recruiting | NCT03088059 |
| Metastatic gastric cancer with FGFR mutation/fusion | II | not yet recruiting | NCT04077255 | ||
| FGFR-positive locally advanced or metastatic urothelial carcinoma | II/III | active, not recruiting | NCT03410693 | ||
| FGFR1–3-positive advanced squamous-cell non-small cell lung cancer | II | recruiting | NCT03762122 | ||
| AZD4547 | FGFR1–3 | FGFR-aberrant advanced refractory solid tumors, lymphomas, or multiple myeloma | II | recruiting | NCT02465060 |
| Muscle-invasive bladder cancer with FGFR mutations/fusions | I | active not recruiting | NCT02546661 | ||
| ER+ breast cancer patients with FGFR1 polysomy (FISH4/5) or gene amplification | I/II | completed | NCT01202591 | ||
| FGFR-aberrant squamous cell lung cancer | II/III | active not recruiting | NCT02965378 | ||
| FGFR1-amplified squamous non-small-cell lung cancer | I/II | completed | NCT01824901 | ||
| Recurrent malignant glioma expressing FGFR–TACC Gene Fusion | I/II | completed | NCT02824133 | ||
| Advanced refractory cancers/lymphomas/multiple myeloma | II | active not recruiting | NCT04439240 | ||
| DEBIO 1347 (CH5183284) | FGFR1–3 | Solid tumors harboring a fusion of FGFR1, FGFR2, or FGFR3 | II | active not recruiting | NCT03834220 |
| FGFR-amplified metastatic breast cancer | I/II | recruiting | NCT03344536 | ||
| FGFR-aberrant advanced solid tumors | I | active not recruiting | NCT01948297 | ||
| Irreversible FGFR inhibitors | |||||
| Futibatinib (TAS-120) | FGFR1–4 | FGFR-aberrant advanced or metastatic solid tumor, advanced or metastatic gastric or gastroesophageal cancer, myeloid or lymphoid neoplasms | II | not yet recruiting | NCT04189445 |
| Advanced cholangiocarcinoma harboring FGFR2 gene rearrangements | III | not yet recruiting | NCT04093362 | ||
| FGF/FGFR aberrant advanced solid tumors | I/II | active, not recruiting | NCT02052778 |
The majority of FGFR selective inhibitors are under evaluation in clinical trials in which only patients harboring FGFR genomic alterations are enrolled (Table 4). In a phase II trial of AZD4547 in patients with advanced cancers with FGFR1–3 aberrations, PRs were observed in 4 of 48 (8%) patients, including 2 patients with FGFR mutations and 2 with FGFR3–TACC3 fusions [63]. The estimated median PFS was 3.4 months. The 6-month PFS rate was low for patients with FGFR amplifications (0%) and for patients carrying FGFR mutations (6%) and higher for patients with FGFR fusions (56%). Three of nine patients with FGFR fusions had a PFS > 10 months [63]. In a multicenter, open label, phase II study on infigratinib in chemotherapy-refractory advanced or metastatic cholangiocarcinoma with FGFR alterations, including 48 FGFR2 fusions, all responsive cases harbored FGFR2 fusions [64]. The RR was 14.8 % and the DCR 75.4% (18.8% and 83.3% for patients with FGFR2 fusions, respectively). Reduced target lesion size in at least one disease evaluation was observed in 36/48 patients with tumors bearing FGFR2 fusions [64].
Only few studies with selective reversible FGFR-TKIs are planned in patients specifically carrying only FGFR fusions, presumably due to the low frequency of these alterations (Table 4).
In this regard, a study exploring the effects of AZD4547 is ongoing in patients with glioma and the FGFR3–TACC3 fusion (ClinicalTrials.gov Identifier: NCT02824133). Infigratinib is under evaluation in a phase III study as first-line treatment for patients with cholangiocarcinoma and FGFR2 gene fusions/translocations (ClinicalTrials.gov Identifier: NCT03773302) and in a phase I study in patients with high-grade glioma and FGFR3–TACC3 translocations (ClinicalTrials.gov Identifier: NCT04424966).
Irreversible inhibitors that covalently bind to a highly conserved P-loop cysteine residue in the ATP pocket of FGFRs have also been developed. Futibatinib is a potent and high selective, irreversible FGFR1–4 inhibitor [65] (Table 4). In a trial exploring futibatinib in previously treated cholangiocarcinoma patients with FGFR alterations, 20/28 patients carrying FGFR2 fusions experienced tumor shrinkage and 7/28 confirmed PR. The ORR was 25% and the DCR 79% [66]. The irreversible FGFR inhibitor futibatinib is currently under evaluation in a phase III clinical study in patients with advanced cholangiocarcinoma harboring FGFR2 gene rearrangements (ClinicalTrials.gov Identifier: NCT04093362).
7. Conclusions and Perspectives
Increasing evidence suggests that the FGFR system plays an important role in cancer development and progression. However, the results of clinical trials have clearly demonstrated that only tumors carrying genetic alterations of the FGFRs such as mutations or fusions might respond to treatment with FGFR inhibitors, at least when used as single agents. In this respect, targeted therapies for FGFR-aberrant tumors may offer an effective therapeutic strategy in some cancer types, such as cholangiocarcinoma, which is often diagnosed in advanced stages when only palliative treatment is available [67]. In the last years, the rapid improvement in the development of drugs targeting FGFR alterations, including fusions, combined with the availability of ever more efficient diagnostic tests, allowed the selection of patients who might benefit from FGFR inhibitors. However, some issues should be considered, such as the need of adequate tools for the detection of FGFR genetic alterations, the identification of the mechanisms of resistance to FGFR inhibitors and the possibility of performing clinical trials specifically for patients with rare alterations.
FGFR fusions are relatively rare genetic alterations. The introduction in clinical diagnostics of NGS panels for comprehensive genomic profiling allowed significant improvement in the detection of FGFR alterations [31,33,53]. However, most available targeted sequencing panels for the detection of FGFR fusions have some limits as we discussed in this review. In addition, lack of tumor tissue is still a limit to perform genomic profiling of patients with advanced disease. In this respect, analysis of circulating cell-free DNA (cfDNA) might represent an alternative approach in patients with no tissue available for genomic profiling [68]. Different targeted sequencing panels have been developed that allow the analysis of a large number of genes, including FGFRs, in cfDNA. Analysis of cfDNA for the detection of FGFR fusions might also serve as a non-invasive tool for monitoring patients undergoing FGFR-targeted therapies and for the identification of biomarkers of resistance. However, some technical issues, such as the low recovery of cfDNA, the low fraction of circulating tumor DNA (<0.1% to 50%) in cfDNA, the short half-life, and the high grade of fragmentation, should be resolved for implementing the clinical validity and utility of this approach [69].
Despite the encouraging results of FGFR inhibitors in clinical trials, mechanisms of acquired resistance with the occurrence of secondary mutations have been described, thus limiting the duration of the response. In particular, in three fusion-positive patients with cholangiocarcinoma treated with BGJ398, the emergence of a secondary FGFR2 kinase domain mutation in one patient and multiple FGFR2 mutations in the remaining two patients was observed [70]. Interestingly, one FGFR2 point mutation (p.V564F) was identified in all patients, suggesting a relevant role of this genomic alteration in the resistance to anti-FGFR agents [70]. A recent study in patients with fusion-positive intrahepatic cholangiocarcinoma who progressed on BGJ398 or Debio1347 revealed that treatment with the FGFR irreversible inhibitor futibatinib might overcome the acquired resistance to FGFR reversible inhibitors [71]. Prospective studies in a large population of patients might confirm these findings.
Finally, it is important to consider that FGFR fusions occur at low frequency in human tumors [17]. The clinical development of targeted agents directed against rare alterations in a specific tumor type is difficult, due to the small number of patients who can be included in clinical studies. Basket trials, in which a sufficient number of patients with specific genetic alterations can be enrolled, regardless of the tumor type, are required, in order to study the significance of these alterations in a larger population and to offer a personalized treatment to patients carrying these rare genomic aberrations. In this respect, clinical trials are ongoing to explore the agnostic role of FGFR fusions as marker of response to drugs targeting the FGFR kinase.
In conclusion, the awareness that FGFR alterations, including fusions, play an important role in cancer has greatly enhanced the clinical development of FGFR inhibitors together with the improvement of NGS-based molecular tests. The design of basket trials might significantly improve the approval of FGFR agents for patients carrying FGFR fusions.
Author Contributions
Conceptualization, A.D.L. and N.N.; Writing—Original Draft Preparation, A.D.L. and N.N.; Writing—Review & Editing, R.E.A., A.M.R., M.R.M., C.E., C.S., F.I., G.N.; Supervision, N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health: M4/9 (A.D.L.) and M4/2 (N.N.)
Conflicts of Interest
N.N. reports personal fees from MSD, grants and personal fees from Qiagen, grants and personal fees from Biocartis, personal fees from Incyte, grants and personal fees from Roche, grants and personal fees from BMS, grants and personal fees from MERCK, grants and personal fees from Thermofisher, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Astrazeneca, personal fees from Sanofi, personal fees from Eli Lilly, personal fees from Bayer, personal fees from ArcherDX, grants and personal fees from Illumina, and personal fees from Amgen, outside the submitted work. The other authors declare no conflict of interest.
References
- 1.Babina I.S., Turner N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 2.Goetz R., Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat. Rev. Clin. Oncol. 2019;16:105–122. doi: 10.1038/s41571-018-0115-y. [DOI] [PubMed] [Google Scholar]
- 4.Mertens F., Johansson B., Fioretos T., Mitelman F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer. 2015;15:371–381. doi: 10.1038/nrc3947. [DOI] [PubMed] [Google Scholar]
- 5.Schram A.M., Chang M.T., Jonsson P., Drilon A. Fusions in solid tumours: Diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017;14:735–748. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beenken A., Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinina J., Dutta K., Ilghari D., Beenken A., Goetz R., Eliseenkova A.V., Cowburn D., Mohammadi M. The alternatively spliced acid box region plays a key role in FGF receptor autoinhibition. Structure. 2012;20:77–88. doi: 10.1016/j.str.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sleeman M., Fraser J., McDonald M., Yuan S., White D., Grandison P., Kumble K., Watson J.D., Murison J.G. Identification of a new fibroblast growth factor receptor, FGFR5. Gene. 2001;271:171–182. doi: 10.1016/S0378-1119(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 9.Trueb B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell. Mol. Life Sci. 2011;68:951–964. doi: 10.1007/s00018-010-0576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang L., Steinberg F., Trueb B. Receptor FGFRL1 acts as a tumor suppressor in nude mice when overexpressed in HEK 293 Tet-On cells. Oncol. Lett. 2016;12:4524–4530. doi: 10.3892/ol.2016.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzmann K., Grunt T., Heinzle C., Sampl S., Steinhoff H., Reichmann N., Kleiter M., Hauck M., Marian B. Alternative Splicing of Fibroblast Growth Factor Receptor IgIII Loops in Cancer. J. Nucleic Acids. 2012;2012:950508. doi: 10.1155/2012/950508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaishi S., Sawada M., Morita Y., Seno H., Fukuzawa H., Chiba T. Identification of a novel alternative splicing of human FGF receptor 4: Soluble-form splice variant expressed in human gastrointestinal epithelial cells. Biochem. Biophys. Res. Commun. 2000;267:658–662. doi: 10.1006/bbrc.1999.2010. [DOI] [PubMed] [Google Scholar]
- 13.Ezzat S., Zheng L., Yu S., Asa S.L. A soluble dominant negative fibroblast growth factor receptor 4 isoform in human MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2001;287:60–65. doi: 10.1006/bbrc.2001.5546. [DOI] [PubMed] [Google Scholar]
- 14.Olsen S.K., Garbi M., Zampieri N., Eliseenkova A.V., Ornitz D.M., Goldfarb M., Mohammadi M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J. Biol. Chem. 2003;278:34226–34236. doi: 10.1074/jbc.M303183200. [DOI] [PubMed] [Google Scholar]
- 15.Goetz R., Beenken A., Ibrahimi O.A., Kalinina J., Olsen S.K., Eliseenkova A.V., Xu C., Neubert T.A., Zhang F., Linhardt R.J., et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo L.H., Nelson K.N., Meyer A.N., Donoghue D.J. Functions of Fibroblast Growth Factor Receptors in cancer defined by novel translocations and mutations. Cytokine Growth Factor Rev. 2015;26:425–449. doi: 10.1016/j.cytogfr.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Helsten T., Elkin S., Arthur E., Tomson B.N., Carter J., Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016;22:259–267. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 18.The AACR Project GENIE Consortium AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lew E.D., Furdui C.M., Anderson K.S., Schlessinger J. The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci. Signal. 2009;2:ra6. doi: 10.1126/scisignal.2000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson D.C., Hurst C.D., Knowles M.A. Knockdown by shRNA identifies S249C mutant FGFR3 as a potential therapeutic target in bladder cancer. Oncogene. 2007;26:5889–5899. doi: 10.1038/sj.onc.1210399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles M.A. Role of FGFR3 in urothelial cell carcinoma: Biomarker and potential therapeutic target. World J. Urol. 2007;25:581–593. doi: 10.1007/s00345-007-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor J.G.t., Cheuk A.T., Tsang P.S., Chung J.Y., Song Y.K., Desai K., Yu Y., Chen Q.R., Shah K., Youngblood V., et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J. Clin. Investig. 2009;119:3395–3407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futami T., Kawase T., Mori K., Asaumi M., Kihara R., Shindoh N., Kuromitsu S. Identification of a novel oncogenic mutation of FGFR4 in gastric cancer. Sci. Rep. 2019;9:14627. doi: 10.1038/s41598-019-51217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker B.C., Engels M., Annala M., Zhang W. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J. Pathol. 2014;232:4–15. doi: 10.1002/path.4297. [DOI] [PubMed] [Google Scholar]
- 25.Shi E., Chmielecki J., Tang C.M., Wang K., Heinrich M.C., Kang G., Corless C.L., Hong D., Fero K.E., Murphy J.D., et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J. Transl. Med. 2016;14:339. doi: 10.1186/s12967-016-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Stefano A.L., Fucci A., Frattini V., Labussiere M., Mokhtari K., Zoppoli P., Marie Y., Bruno A., Boisselier B., Giry M., et al. Detection, Characterization, and Inhibition of FGFR-TACC Fusions in IDH Wild-type Glioma. Clin. Cancer Res. 2015;21:3307–3317. doi: 10.1158/1078-0432.CCR-14-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh D., Chan J.M., Zoppoli P., Niola F., Sullivan R., Castano A., Liu E.M., Reichel J., Porrati P., Pellegatta S., et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephens P.J., McBride D.J., Lin M.L., Varela I., Pleasance E.D., Simpson J.T., Stebbings L.A., Leroy C., Edkins S., Mudie L.J., et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462:1005–1010. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross J.S., Wang K., Khaira D., Ali S.M., Fisher H.A., Mian B., Nazeer T., Elvin J.A., Palma N., Yelensky R., et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer. 2016;122:702–711. doi: 10.1002/cncr.29826. [DOI] [PubMed] [Google Scholar]
- 30.Wang R., Wang L., Li Y., Hu H., Shen L., Shen X., Pan Y., Ye T., Zhang Y., Luo X., et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin. Cancer Res. 2014;20:4107–4114. doi: 10.1158/1078-0432.CCR-14-0284. [DOI] [PubMed] [Google Scholar]
- 31.Qin A., Johnson A., Ross J.S., Miller V.A., Ali S.M., Schrock A.B., Gadgeel S.M. Detection of Known and Novel FGFR Fusions in Non-Small Cell Lung Cancer by Comprehensive Genomic Profiling. J. Thorac. Oncol. 2019;14:54–62. doi: 10.1016/j.jtho.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Arai Y., Totoki Y., Hosoda F., Shirota T., Hama N., Nakamura H., Ojima H., Furuta K., Shimada K., Okusaka T., et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 33.Ross J.S., Wang K., Gay L., Al-Rohil R., Rand J.V., Jones D.M., Lee H.J., Sheehan C.E., Otto G.A., Palmer G., et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowery M.A., Ptashkin R., Jordan E., Berger M.F., Zehir A., Capanu M., Kemeny N.E., O’Reilly E.M., El-Dika I., Jarnagin W.R., et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018;24:4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain A., Borad M.J., Kelley R.K., Wang Y., Abdel-Wahab R., Meric-Bernstam F., Baggerly K.A., Kaseb A.O., Al-shamsi H.O., Ahn D.H., et al. Cholangiocarcinoma With FGFR Genetic Aberrations: A Unique Clinical Phenotype. JCO Precis. Oncol. 2018:1–12. doi: 10.1200/PO.17.00080. [DOI] [PubMed] [Google Scholar]
- 36.Borad M.J., Champion M.D., Egan J.B., Liang W.S., Fonseca R., Bryce A.H., McCullough A.E., Barrett M.T., Hunt K., Patel M.D., et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS ONE Genet. 2014;10:e1004135. doi: 10.1371/journal.pgen.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sia D., Losic B., Moeini A., Cabellos L., Hao K., Revill K., Bonal D., Miltiadous O., Zhang Z., Hoshida Y., et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat. Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y.M., Su F., Kalyana-Sundaram S., Khazanov N., Ateeq B., Cao X., Lonigro R.J., Vats P., Wang R., Lin S.F., et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanizaki J., Ercan D., Capelletti M., Dodge M., Xu C., Bahcall M., Tricker E.M., Butaney M., Calles A., Sholl L.M., et al. Identification of Oncogenic and Drug-Sensitizing Mutations in the Extracellular Domain of FGFR2. Cancer Res. 2015;75:3139–3146. doi: 10.1158/0008-5472.CAN-14-3771. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Ding X., Wang S., Moser C.D., Shaleh H.M., Mohamed E.A., Chaiteerakij R., Allotey L.K., Chen G., Miyabe K., et al. Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett. 2016;380:163–173. doi: 10.1016/j.canlet.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hood F.E., Royle S.J. Pulling it together: The mitotic function of TACC3. Bioarchitecture. 2011;1:105–109. doi: 10.4161/bioa.1.3.16518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams S.V., Hurst C.D., Knowles M.A. Oncogenic FGFR3 gene fusions in bladder cancer. Hum. Mol. Genet. 2013;22:795–803. doi: 10.1093/hmg/dds486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carneiro B.A., Elvin J.A., Kamath S.D., Ali S.M., Paintal A.S., Restrepo A., Berry E., Giles F.J., Johnson M.L. FGFR3-TACC3: A novel gene fusion in cervical cancer. Gynecol. Oncol. Rep. 2015;13:53–56. doi: 10.1016/j.gore.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi Y., Akiyama N., Tsukaguchi T., Fujii T., Satoh Y., Ishii N., Aoki M. Mechanism of Oncogenic Signal Activation by the Novel Fusion Kinase FGFR3-BAIAP2L1. Mol. Cancer Ther. 2015;14:704–712. doi: 10.1158/1535-7163.MCT-14-0927-T. [DOI] [PubMed] [Google Scholar]
- 45.Kumar-Sinha C., Kalyana-Sundaram S., Chinnaiyan A.M. Landscape of gene fusions in epithelial cancers: Seq and ye shall find. Genome Med. 2015;7:129. doi: 10.1186/s13073-015-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stam K., Heisterkamp N., Grosveld G., de Klein A., Verma R.S., Coleman M., Dosik H., Groffen J. Evidence of a new chimeric bcr/c-abl mRNA in patients with chronic myelocytic leukemia and the Philadelphia chromosome. N. Engl. J. Med. 1985;313:1429–1433. doi: 10.1056/NEJM198512053132301. [DOI] [PubMed] [Google Scholar]
- 47.Speicher M.R., Carter N.P. The new cytogenetics: Blurring the boundaries with molecular biology. Nat. Rev. Genet. 2005;6:782–792. doi: 10.1038/nrg1692. [DOI] [PubMed] [Google Scholar]
- 48.Cheng L., Zhang S., Wang L., MacLennan G.T., Davidson D.D. Fluorescence in situ hybridization in surgical pathology: Principles and applications. J. Pathol. Clin. Res. 2017;3:73–99. doi: 10.1002/cjp2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurobe M., Kojima T., Nishimura K., Kandori S., Kawahara T., Yoshino T., Ueno S., Iizumi Y., Mitsuzuka K., Arai Y., et al. Development of RNA-FISH Assay for Detection of Oncogenic FGFR3-TACC3 Fusion Genes in FFPE Samples. PLoS ONE. 2016;11:e0165109. doi: 10.1371/journal.pone.0165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L., Lee M.S., Lu H., Oh D.Y., Kim Y.J., Park D., Park G., Ren X., Bristow C.A., Haseley P.S., et al. Analyzing Somatic Genome Rearrangements in Human Cancers by Using Whole-Exome Sequencing. Am. J. Hum. Genet. 2016;98:843–856. doi: 10.1016/j.ajhg.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshihara K., Wang Q., Torres-Garcia W., Zheng S., Vegesna R., Kim H., Verhaak R.G. The landscape and therapeutic relevance of cancer-associated transcript fusions. Oncogene. 2015;34:4845–4854. doi: 10.1038/onc.2014.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Z., Liebers M., Zhelyazkova B., Cao Y., Panditi D., Lynch K.D., Chen J., Robinson H.E., Shim H.S., Chmielecki J., et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 53.Kongpetch S., Jusakul A., Lim J.Q., Ng C.C.Y., Chan J.Y., Rajasegaran V., Lim T.H., Lim K.H., Choo S.P., Dima S., et al. Lack of Targetable FGFR2 Fusions in Endemic Fluke-Associated Cholangiocarcinoma. JCO Glob. Oncol. 2020;6:628–638. doi: 10.1200/GO.20.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham R.P., Barr Fritcher E.G., Pestova E., Schulz J., Sitailo L.A., Vasmatzis G., Murphy S.J., McWilliams R.R., Hart S.N., Halling K.C., et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum. Pathol. 2014;45:1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Churi C.R., Shroff R., Wang Y., Rashid A., Kang H.C., Weatherly J., Zuo M., Zinner R., Hong D., Meric-Bernstam F., et al. Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS ONE. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah R.R., Morganroth J. Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit. Drug Saf. 2015;38:693–710. doi: 10.1007/s40264-015-0300-1. [DOI] [PubMed] [Google Scholar]
- 57.Konecny G.E., Finkler N., Garcia A.A., Lorusso D., Lee P.S., Rocconi R.P., Fong P.C., Squires M., Mishra K., Upalawanna A., et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: A non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015;16:686–694. doi: 10.1016/S1470-2045(15)70159-2. [DOI] [PubMed] [Google Scholar]
- 58.Hahn N.M., Bivalacqua T.J., Ross A.E., Netto G.J., Baras A., Park J.C., Chapman C., Masterson T.A., Koch M.O., Bihrle R., et al. A Phase II Trial of Dovitinib in BCG-Unresponsive Urothelial Carcinoma with FGFR3 Mutations or Overexpression: Hoosier Cancer Research Network Trial HCRN 12-157. Clin. Cancer Res. 2017;23:3003–3011. doi: 10.1158/1078-0432.CCR-16-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzaferro V., El-Rayes B.F., Droz Dit Busset M., Cotsoglou C., Harris W.P., Damjanov N., Masi G., Rimassa L., Personeni N., Braiteh F., et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soria J.C., DeBraud F., Bahleda R., Adamo B., Andre F., Dientsmann R., Delmonte A., Cereda R., Isaacson J., Litten J., et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann. Oncol. 2014;25:2244–2251. doi: 10.1093/annonc/mdu390. [DOI] [PubMed] [Google Scholar]
- 61.Loriot Y., Necchi A., Park S.H., Garcia-Donas J., Huddart R., Burgess E., Fleming M., Rezazadeh A., Mellado B., Varlamov S., et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019;381:338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 62.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., Paulson A.S., Borad M.J., Gallinson D., Murphy A.G., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chae Y.K., Hong F., Vaklavas C., Cheng H.H., Hammerman P., Mitchell E.P., Zwiebel J.A., Ivy S.P., Gray R.J., Li S., et al. Phase II Study of AZD4547 in Patients With Tumors Harboring Aberrations in the FGFR Pathway: Results From the NCI-MATCH Trial (EAY131) Subprotocol W. J. Clin. Oncol. 2020;38:2407–2417. doi: 10.1200/JCO.19.02630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Javle M., Lowery M., Shroff R.T., Weiss K.H., Springfeld C., Borad M.J., Ramanathan R.K., Goyal L., Sadeghi S., Macarulla T., et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018;36:276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalyukina M., Yosaatmadja Y., Middleditch M.J., Patterson A.V., Smaill J.B., Squire C.J. TAS-120 Cancer Target Binding: Defining Reactivity and Revealing the First Fibroblast Growth Factor Receptor 1 (FGFR1) Irreversible Structure. ChemMedChem. 2019;14:494–500. doi: 10.1002/cmdc.201800719. [DOI] [PubMed] [Google Scholar]
- 66.Meric-Bernstam F., Arkenau H., Tran B., Bahleda R., Kelley R., Hierro C., Ahn D., Zhu A., Javle M., Winkler R., et al. Efficacy of TAS-120, an irreversible fibroblast growth factor receptor (FGFR) inhibitor, in cholangiocarcinoma patients with FGFR pathway alterations who were previously treated with chemotherapy and other FGFR inhibitors. Ann. Oncol. 2018;29:v100. doi: 10.1093/annonc/mdy149. [DOI] [Google Scholar]
- 67.Lamarca A., Barriuso J., McNamara M.G., Valle J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020;73:170–185. doi: 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Normanno N., Cervantes A., Ciardiello F., De Luca A., Pinto C. The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treat. Rev. 2018;70:1–8. doi: 10.1016/j.ctrv.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Heitzer E., Haque I.S., Roberts C.E.S., Speicher M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019;20:71–88. doi: 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 70.Goyal L., Saha S.K., Liu L.Y., Siravegna G., Leshchiner I., Ahronian L.G., Lennerz J.K., Vu P., Deshpande V., Kambadakone A., et al. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017;7:252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goyal L., Shi L., Liu L.Y., Fece de la Cruz F., Lennerz J.K., Raghavan S., Leschiner I., Elagina L., Siravegna G., Ng R.W.S., et al. TAS-120 Overcomes Resistance to ATP-Competitive FGFR Inhibitors in Patients with FGFR2 Fusion-Positive Intrahepatic Cholangiocarcinoma. Cancer Discov. 2019;9:1064–1079. doi: 10.1158/2159-8290.CD-19-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]