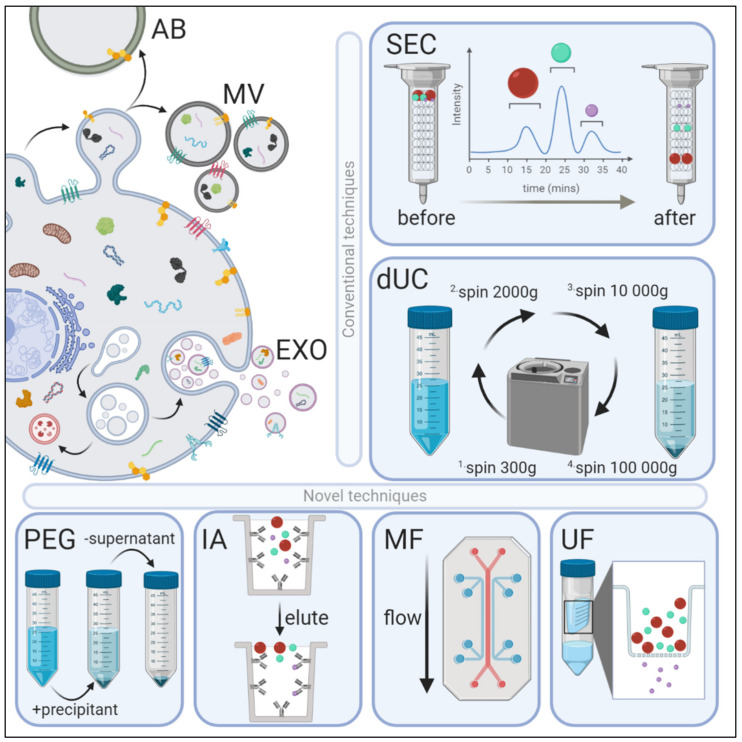
Figure 1.
Extracellular vesicle (EV) biogenesis, subpopulations, and conventional and novel methods of exosome isolation. EVs are categorized into three main types depending on their site of origin, density, expression of markers, and/or size. Apoptotic bodies (AB) are released by the blebbing of an apoptotic cell membrane (500–5000 nm); microvesicles (MV) are shed from the outward budding of the plasma membrane (100–1000 nm); and exosomes (EXO) are formed when multivesicular bodies fuse to the plasma membrane and release intraluminal vesicles (30–150 nm). EVs have variable: (1) protein expression profiles—EXOs are enriched with Fliotillin-1, ALIX, TSG101, CD81, CD63 and CD9 proteins, whereas MVs preferentially express MMP2 and ARF6; (2) lipidomic profiles—MVs are enriched with phosphatidylserine and cholesterol, EXOs with sphingomyelin and ceramide, and ABs by phosphatidylserine; and (3) distinct genomic and transcriptomic luminal cargo. Conventional methods of EV isolation include size-exclusion chromatography (SEC) and differential ultracentrifugation (dUC). SEC uses biofluids as a mobile phase against a porous stationary phase to differentially elute molecules with an inverse speed relation to their size—in other words, larger particles will elute first, followed by smaller vesicles that will enter and flow through the pores resulting in a longer path and thus increased elution time. dUC relies on the separation of EV subpopulations via gradually higher acceleration rates. More novel exosomal techniques also exist. Poly-ethylene glycol (PEG)-based precipitation uses a solution to facilitate a polymer-entrapped vesicle aggregate in large numbers. Immunoaffinity (IA) capture uses antibodies targeted against exosomal surface proteins to isolate specific vesicle population. Microfluidics (MF) technology uses chips with specific antibody-mediated binding to capture exosomes efficiently. Ultrafiltration (UF) is dependent on a filter of specific pore size that creates a vesicle-rich filtrate specific to the desired size.