Key Points
No meaningful responses were observed when axicabtagene ciloleucel was used for progression after a different CD19-directed CAR T cell.
Further research is needed to understand how to sequence cell-based therapies for relapsed/refractory large B-cell lymphomas.
Introduction
CD19-targeting chimeric antigen receptor T-cells (CAR-T) is efficacious for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL).1-3 In addition to 2 US Food and Drug Administration–approved CAR-T agents (axicabtagene ciloleucel [axi-cel], tisagenlecleucel), investigational CD19 CAR-T products show promise in clinical trials. Long-term remissions are observed in 30% to 40% of patients; however, treatment options are limited for patients whose disease fails to respond to or relapses after CD19 CAR-T. We previously reported an overall survival of approximately 5 months after post–CAR-T progression.4 One option for salvage includes treatment with an alternative CD19-targeting CAR-T product. Although both axi-cel and tisagenlecleucel use single-chain variable fragments (scFvs) derived from the FMC63 murine CD19-targeting monoclonal antibody, using an alternative CAR-T could be beneficial given differences in cell manufacturing and construct (costimulation with 4-1BB vs CD28, selection of scFv)5.
We administered axi-cel off-study in 3 patients exhibiting relapsed/refractory DLBCL after treatment with tisagenlecleucel or investigational CD19 CAR-T with 4-1BB costimulation. The study was approved by the Fred Hutchinson Cancer Research Center institutional review board. Summary of clinical information and selected laboratory/inflammatory markers are presented in Table 1 and Figure 1.
Table 1.
Characteristics of first and second CAR-T infusions, responses, and outcomes
| Patient | Age, y | No. prior treatments and regimen | Stage | IPI | Cell of origin | Cytogenetics | First CAR-T infusion | Second CAR-T infusion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First CAR-T type and cell dose | Pre-CAR-T largest lymph node, cm | Pre-CAR-T LDH (<210 U/L) | CRS | ICANS | Response | Time to second CAR-T, d | Interim treatments | Second CAR-T type and cell dose | Pre-CAR-T largest lymph node, cm | Pre-CAR-T LDH (<210 U/L) | CRS | ICANS | Response | Outcome (days from second CAR-T) | |||||||
| 1 | 78 | (2) R-CHOP GCD | IV | 3 | non-GCB | Neg MYC rearrangement | Experimental CAR-T (4-1-BB)/fully human scFv/2 × 106/kg | 1.7 | 117 | — | — | PR → PD on day 90 | 193 | RT | axi-cel/≤2 × 108 | 4 | 120 | 1 | — | CR → PD on day 160 | Died (180) |
| 2 | 33 | (4) R-CHOP R-ICE GEMOX PembroR | IV | 2 | GCB | Neg MYC rearrangement | Tisagenlecleucel (4-1-BB)/2 × 106 to 2.5 × 108 | 6.2 | 158 | — | — | PD | 152 | RT | axi-cel/≤2 × 108 | 8.2 | 115 | 1 | — | PD | Died (303) |
| 3 | 64 | (3) EPOCH R-ICE R-GCD | IV | 3 | — | MYC and BCL-2 rearrangement | Experimental CAR-T (4-1-BB)/2 × 106/kg | 2.1 | 191 | — | — | PD | 92 | RT | axi-cel/≤2 × 108 | 1.6 | 262 | — | — | PD | Died (282) |
CR, complete response; CRS, cytokine release syndrome; EPOCH, etoposide prednisone vincristine cyclophosphamide doxorubicin; GCB, germinal center B-cell like; GCD, gemcitabine carboplatin dexamethasone; GEMOX, gemcitabine oxaliplatin; ICANS, immune effector cell-associated neurotoxicity syndrome; IPI, International Prognostic Index; LDH, lactate dehydrogenase; LN, lymph node; PD, progressive disease; PR, partial response; PembroR, pembrolizumab rituximab; RT, radiotherapy; R-CHOP, rituximab cyclophosphamide doxorubicin vincristine prednisone; R-GCD, rituximab gemcitabine carboplatin etoposide; R-ICE, rituximab ifosfamide carboplatin etoposide.
Figure 1.
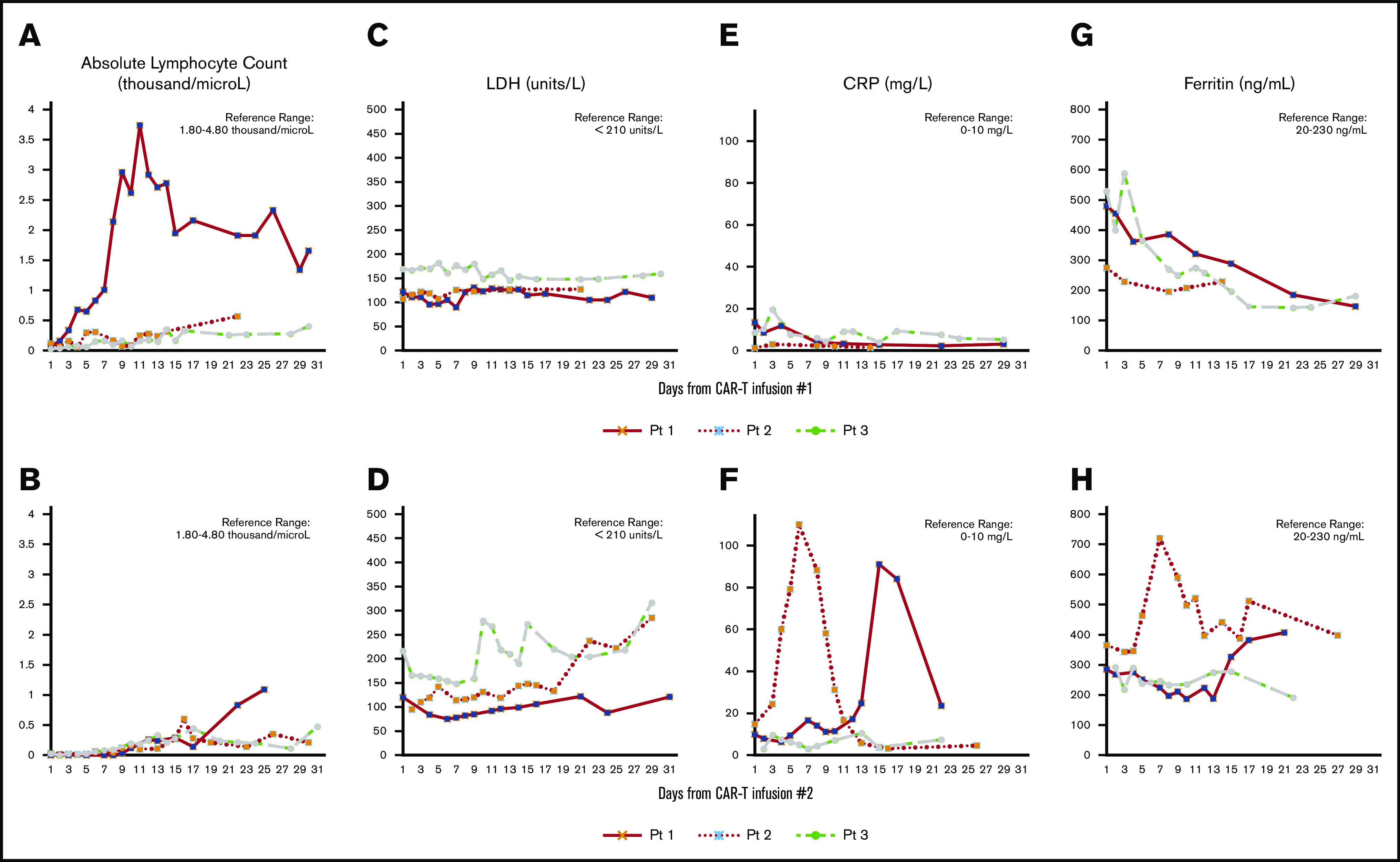
Post–CAR-T biomarkers. Laboratory/immune markers after CAR-T infusion 1 (A,C,E,G) and CAR-T infusion 2 (B,D,F,H). CRP, C-reactive protein; LDH, lactate dehydrogenase.
Case description
Patient 1
A 78-year-old man had follicular lymphoma (grade 3B) that transformed to DLBCL. After relapsing from 2 prior treatments (Table 1), biopsy of a pelvic soft tissue mass before CAR-T revealed CD19-positive DLBCL via flow cytometry (FCM). He received fludarabine-cyclophosphamide lymphodepletion followed by infusion of T cells expressing a CAR with an anti-CD19 fully human scFv and 4-1BB costimulation (#NCT03103971). He tolerated treatment without cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS); however, at day +30, positron emission tomography-computed tomography (PET-CT) revealed 2 mildly fluorine-18-fluorodeoxyglucose (FDG)-avid right level 1B nodes (standardized uptake value [SUV] 2.5, 2.9), present in prior imaging, felt to be equivocal. These cervical nodes regressed, and he was monitored without clinical evidence for relapse. Unfortunately, day +90 PET-CT showed a new FDG-avid abdominal mass (4 × 3 cm; SUV 28) with core biopsy confirming CD19-positive DLBCL via FCM. Of note, the prior cervical nodes showed no metabolic activity. He received bridging radiation therapy (RT) to the abdominal mass (900 cGy) 18 days before axi-cel. Pretreatment PET-CT showed minimal improvement in the irradiated abdominal mass (3.8 × 3.0 cm; SUV 20) and questionable new focal FDG avidity in the spleen (SUV 4.3). He received axi-cel after lymphodepletion with fludarabine-cyclophosphamide 193 days after his first CAR-T, developing grade 1 CRS (fever) but no ICANS and never requiring tocilizumab or corticosteroids. Restaging PET-CT on day +30 showed resolution in the abdomen and spleen (Deauville 1), consistent with a complete response (CR). Repeat CT on day +90 showed continued remission, but the day +160 CT performed for worsening abdominal pain revealed progressive disease (PD). Further biopsies were not pursued, and he died in hospice 180 days after treatment with axi-cel.
Patient 2
A 33-year-old woman had DLBCL. After relapsing from 4 prior treatments (Table 1), a mediastinal mass biopsy revealed low-CD19-expression DLBCL via FCM. In addition, there was PDL-1 expression with Ki67 90% via immunohistochemistry. She received standard lymphodepletion followed by tisagenlecleucel as first CAR-T. She did not develop any CRS or ICANS after tisagenlecleucel, and day +30 PET-CT indicated stable disease with decreased size and FDG avidity of index lesions. Day +90 CT revealed PD. Biopsy of an enlarging left adrenal mass confirmed DLBCL positive for CD19 and PDL-1 expression and negative for CD20 expression via immunohistochemistry. Given a degenerate sample, FCM was unable to detect any abnormal B- or T-cell populations. While awaiting second CAR-T administration with axi-cel, she developed increasing dyspnea on exertion and tachycardia, for which bridging RT was used (900 cGy) to the mediastinal mass. Two days after RT, she received standard lymphodepletion and axi-cel, which was 152 days after tisagenlecleucel. She developed grade 1 CRS (fever) but had no evidence of ICANS. On day +21 after axi-cel, she developed worsening fatigue and night sweats and was found to have PD on PET-CT in all areas except for the anterior mediastinal mass, which showed a mild decrease in size. A repeat biopsy of the left adrenal mass confirmed CD19-positive, CD20-negative DLBCL via immunohistochemistry and FCM. Subsequent treatments included a phase-1 investigational agent (PD); RT to renal lesions (1200 cGy); venetoclax (PD); and polatuzimab-vedotin/bendamustine for 5 cycles (PD). She transitioned to hospice and died of PD 303 days after treatment with axi-cel.
Patient 3
A 64-year-old man had double-hit FL/DLBCL for 8 years before referral for CAR-T therapy. After relapsing from 3 prior treatments (Table 1), a submandibular lymph node biopsy revealed a CD19-positive high-grade B-cell lymphoma via immunohistochemistry and FCM, with MYC and BCL-2 rearrangement on fluorescence in situ hybridization. He received an experimental CD19-targeted product (second generation with 4-1BB as a costimulatory molecule) in combination with an anti–PD-L1 antibody (NCT02706405). He had no CRS or ICANS after treatment, but imaging on day +30 revealed PD in multiple areas, requiring palliative RT to an enlarging cervical mass (4000 cGy). Repeat biopsies were not performed, and he received standard lymphodepletion followed by axi-cel 92 days after prior CAR-T. On day +6 after axi-cel infusion, PD was again noted in the previously irradiated cervical region. Right cervical lymph node biopsy reconfirmed CD19-positive DLBCL via immunohistochemistry and FCM, and he again received palliative RT (5400 cGy). He did not experience CRS or ICANS after axi-cel. Subsequent treatments included duvelisib (PD); polatuzumab-vedotin/bendamustine/R (PD); RT to the left parotid (5400 cGy); and ibrutinib (2 weeks). The patient died of PD 282 days after treatment with axi-cel.
Methods
Our 3 patient experience showed minimal toxicities when commercial axi-cel was administered after relapse following 3 different CD19-targeted CAR-T agents, all using 4-1BB costimulation. We did observe a transient 2 to 3 times greater than upper limit of normal values for C-reactive protein and ferritin after axi-cel (Figure 1) for patients 1 and 2, both whom developed grade 1 CRS; none developed ICANS. However, no long-term remission after axi-cel administration was observed, with PD as best response in patients 2 and 3. Although patient 1 garnered a CR of 5-month duration after axi-cel, he also received RT to a dominant site of disease shortly before axi-cel administration, making it unclear which modality ultimately rendered a transient CR. The reasons for a lack of durable response have not been established, as all were treated off protocol with no correlative data captured. CD19 expression, however, was reported from 2 of 3 patients before axi-cel infusion: 1 via FCM (patient 1) and 1 via immunohistochemistry (patient 2). Although immunohistochemistry may be unreliable for distinguishing between intracellular and membranous antigen expression and more difficult to quantify than FCM, it appears that CD19 antigenicity was still present. Furthermore, patients 2 and 3 again showed CD19 expression at time of progression after axi-cel, both via FCM and immunohistochemistry, making antigen escape an unlikely dominant reason for axi-cel failure. It is intriguing that patient 1, achieving CR after axi-cel, received his first CAR-T product, which incorporated a different scFv from that contained in axi-cel, whereas patients 2 and 3 with PD each received CAR-T products harboring scFvs derived from the same murine monoclonal antibody as axi-cel. One could hypothesize that immune-mediated rejection may have occurred on axi-cel infusion for patients 2 and 3 because no response ever occurred, whereas an immune-suppressing tumor microenvironment, poor T-cell function, or possible antigen downregulation may have contributed in patient 1. We did not capture T-cell expansion data, but poor expansion after axi-cel is possible, considering the lack of any heightened or sustained inflammatory response. However, in trending the absolute lymphocyte count after axi-cel, patient 1, who garnered a CR, was also the only patient to show a trend toward lymphocyte recovery.
Results and discussion
We recently reported our experience with repeat infusions of the same investigational CD19 CAR-T product (#NCT01865617) and showed that higher cell dose in the second infusion and more intensive lymphodepletion were associated with better outcomes in some patients.5,6 Additional studies will determine if axi-cel can, or should, be used in the post-CD19 CAR-T failure setting, particularly in patients previously receiving a construct incorporating the same scFv. Our experience would suggest a lack of efficacy for the reasons mentioned above, in addition to other mechanisms of resistance not explored, such as PDL-1 expression at time of progression, development of human antimurine antibodies, and/or poor T-cell expansion after axi-cel administration. Clinical trials with CAR-Ts targeting an alternative antigen, bivalent CAR-Ts targeting multiple antigens, and administration of 1 or more CAR-Ts targeting different antigens deserve continued investigation after CD19-targeted CAR-T failure.
Footnotes
For data, please contact the corresponding author at mshadman@fhcrc.org.
Authorship
Contribution: M.S. and V.A.C. wrote the manuscript; and V.A.C., A.K.G., J.G., Y.D.T., C.J.T., D.G.M., and M.S. edited the manuscript.
Conflict-of-interest disclosure: A.K.G. reports grants and nonfinancial support from Teva, Bristol-Myers Squibb, Merck, Takeda, TG Therapeutics, and Effector; grants, personal fees, and nonfinancial support from Seattle Genetics, Pfizer, Janssen, Gilead, Spectrum, Amgen and Incyte; personal fees from Aptevo, BRIM Bio, Seattle Genetics, IgM, ADC Therapeutics, and Sanofi. C.J.T. receives research funding from Juno Therapeutics, Nektar Therapeutics, Minerva, TCR2, and AstraZeneca; is a member of scientific advisory boards and has options in Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, and ArsenalBio; serves on scientific advisory boards for T-CURX and Century Therapeutics; has served on advisory boards for Nektar Therapeutics, Allogene, Kite/Gilead, Novartis, Humanigen, PACT Pharma, Amgen, and AstraZeneca; and has patents licensed to Juno Therapeutics. D.G.M. receives research funding, paid to his institution, from Kite Pharma, a Gilead company, and Celgene, a Bristol-Myers Squibb company; receives research funding, paid to his institution, from and has patents licensed or pending with Juno Therapeutics, a Celgene/Bristol-Myers Squibb company; has participated in advisory board and/or protocol-specific data monitoring committee meetings for BioLine RX, Kite Pharma, Gilead, Novartis, Juno Therapeutics, Celgene, and Pharmacyclics and received honoraria; and is a member of the A2 Biotherapeutics Scientific Advisory Board, has received honoraria, and has stock options. M.S. received research funding from Mustang Bio, Celgene, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, Beigene, Acerta Pharma, and Merck, and has served on advisory boards or as a consultant for AbbVie, Genentech, AstraZeneca, Sound Biologics, Verastem, ADC Therapeutics, BMS, and Atara Biotherapeutics. The remaining authors declare no competing financial interests.
Correspondence: Mazyar Shadman, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-396, Seattle, WA 98109; e-mail: mshadman@fredhutch.org.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 3.Turtle CJ, Hay KA, Hanafi LA, et al. . Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow VA, Gopal AK, Maloney DG, et al. . Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94(8):E209-E213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezerra ED, Gauthier J, Hirayama AV, et al. . Factors associated with response, CAR-T cell in vivo expansion, and progression-free survival after repeat infusions of CD19 CAR-T cells [abstract]. Blood. 2019;134(suppl 1). Abstract 201. [Google Scholar]
- 6.Hirayama AV, Gauthier J, Hay KA, et al. . The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]


