Scheme 2.
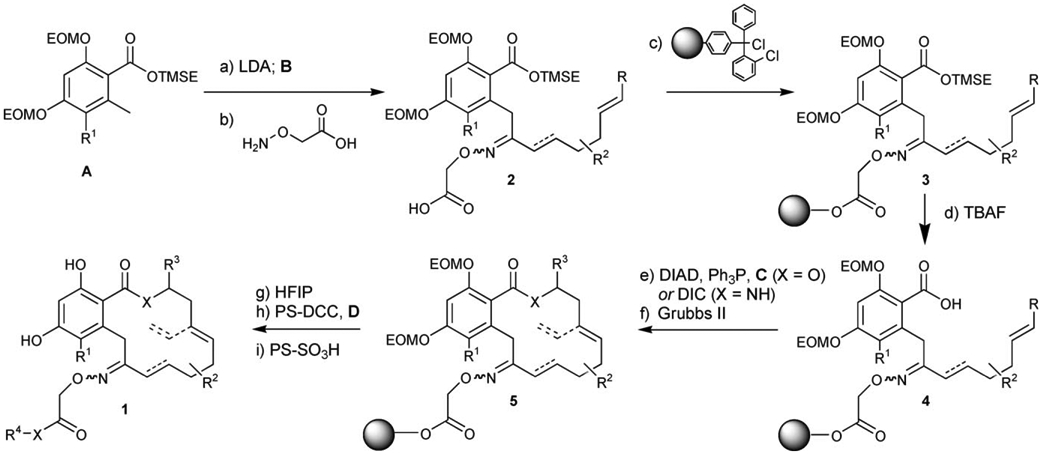
Reagents and conditions: a) LDA (2.0 equiv), B (0.9 equiv), THF, −78°C, 20 min, 50–85%; b) H2NOCH2CO2H (5.0 equiv), 40°C, py, 24–48 h, 85–95%; c) PS-ClTr-Cl (3.0 equiv), EtiPr2N (6.0 equiv), CH2Cl2, 23°C, 24 h; then AcOH (20 equiv), 23°C, 24 h; d) TBAF (4.0 equiv), 23°C 4 h; e) C (5.0 equiv), Ph3P (2.0 equiv), DIAD (2.0 equiv), toluene, 23°C, 12 h (for X=O) or C (2.0 equiv), DIC (5.0 equiv), 4-DMAP (5 mol%), CH2Cl2, 23°C, 12 h (for X=NH); f) Grubbs’ II (0.06 equiv), CH2Cl2, 120°C microwave, 3×45 min; g) HFIP/CH2Cl2 (1/4), 23°C, 3 h, 20–30% over five steps; h) PS-DCC (3.0 equiv), DMAP (cat), D (2.0 equiv), 23°C 72 h; i) PS-SO3H (10 equiv), MeOH, 23°C 4 h, >75% for two steps (>90% conversion).
