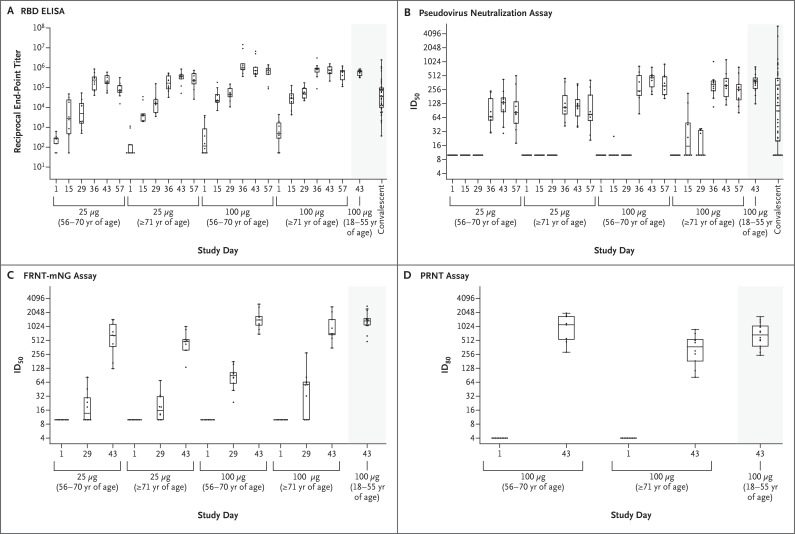
Figure 2. SARS-CoV-2 Antibody-Binding and Neutralization Responses.
Shown are reciprocal end-point binding IgG titers on receptor-binding domain (RBD) enzyme-linked immunosorbent assay (ELISA) (Panel A), along with titers to 614D on the pseudovirus neutralization assay at a 50% inhibitory dilution (ID50) (Panel B), on the focus reduction neutralization test mNeonGreen assay (FRNT-mNG ID50) (Panel C), and on plaque-reduction neutralization testing (PRNT80) (Panel D).2 PRNT80 results were available only for the participants who had received the 100-μg dose of the mRNA-1273 vaccine on days 1 and 43. Boxes denote interquartile ranges, and horizontal bars denote median end-point titers. Whisker end points denote the maximum and minimum values below or above the median at 1.5 times the interquartile range. The shaded portion on the right side of each panel indicates one or two categories of reference values: antibody titers from 41 controls who had donated convalescent serum (Panels A and B) and antibody titers from participants between the ages of 18 and 55 years who had received the 100-μg dose of mRNA-1273 (Panels A through D).2