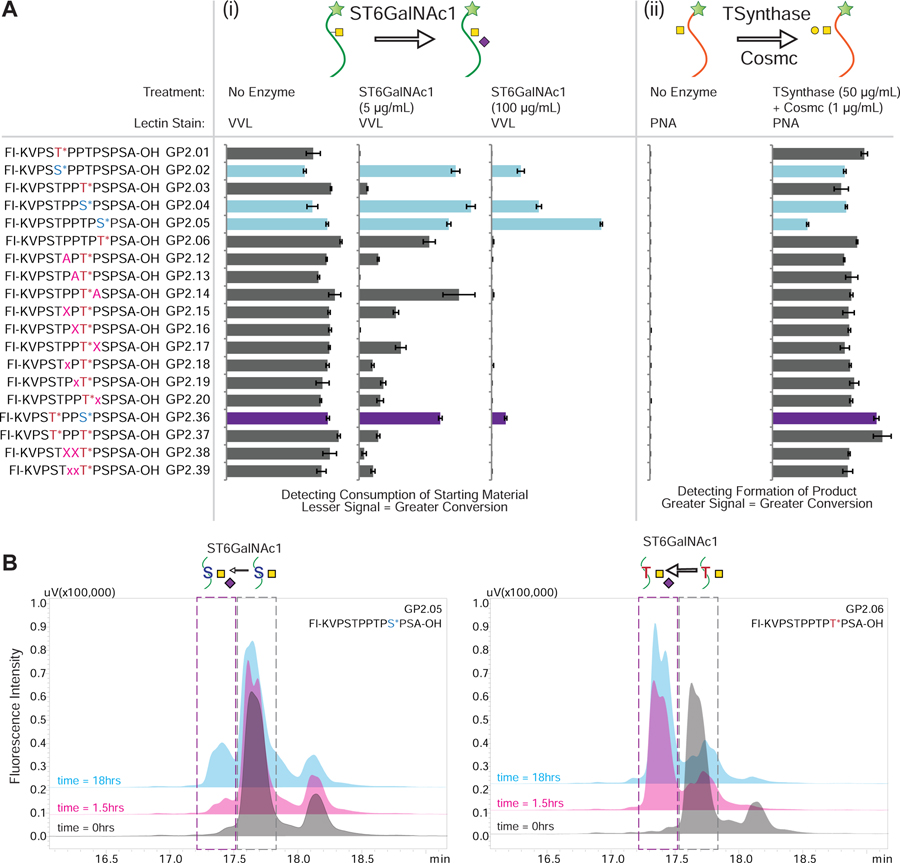
Figure 5.
(A) On-array glycosylation using glycosyltransferase enzymes. The sequences of all the relevant IgA1 glycopeptides containing GalNAcα1- glycosylation are shown on the left where the * indicating the sites of glycosylation and the GP IDs are provided next to the sequence. The figure demonstrates how the binding pattern of lectins change when the array is treated with different glycosyltransferases either (i) ST6GalNAc1: The readout is using the binding of VVL lectin to the GalNAc on the peptides. As the reaction proceeds further, the binding of VVL lectin decreases as the product of the reaction is Neu5Acα2–6GalNAcα1- glycosylation, which is not recognized by the lectin. It was observed that the serine containing glycopeptides (highlighted in light blue or purple for the mixed glycosylation) are more resistant to sialylation by the enzyme. (ii) T-synthase: The readout is using PNA lectin which detects formation of the T-antigen (Galβ1–3GalNAcα1-S/T). Since PNA binds to the product of the reaction we see an increase in the glycosylation for all GalNAc containing peptides. As can be seen, T-synthase is not as selective about glycosylation. The data was normalized to the tallest peak in the dataset to highlight the differences clearly. Error bars represent +/− 1 standard deviation. Full array data is provided in Figure S6. (B) In-solution glycosylation using glycosyltransferase ST6GalNAc1. GP2.05 and GP2.06 were treated with ST6GalNAc1 in solution and the reaction was monitored over time using HPLC and MALDI-MS. As can be seen from the HPLC profiles, GP2.05 which contains GalNAcα1-Ser is resistant to conversion and shows little conversion (<25%) after 18 hours. In comparison GP2.05 contains GalNAcα1-Thr is converted (>80%) within 1.5 hours.