Summary
Introduction
Gestational weight gain (GWG) and postpartum weight retention (PPWR) are significant, potentially modifiable, contributors to women's future weight and health trajectories. There is a need for feasible and patient‐centered (i.e., convenient, remotely‐delivered, technology‐enhanced, and accessible through the prenatal care setting) behavioural interventions that limit GWG and PPWR. This study tests the feasibility and acceptability of a remotely‐delivered behavioural health coaching intervention to limit gestational weight gain and postpartum weight retention.
Methods
Pregnant women (11–16 weeks gestation) were recruited from two prenatal clinics and randomized to the active intervention or health education comparison group. Completion of the program was monitored and perceived helpfulness was rated (0–100).
Results
Twenty‐six women were randomized (n = 13 per arm; mean age = 31.6 years, SD = 3.6; mean BMI = 26.7 kg/m2, SD = 7.4). Participants completed a median of 18 coaching calls and 16/19 learning activities during pregnancy, and a median of 6 calls and 5/6 learning activities postpartum. They logged weights at least once/week for a median of 36/38 expected weeks and tracked daily calories and exercise for a median of 154/266 days and 72/266 days, respectively. Median (Q1, Q3) helpfulness ratings of the program during pregnancy were 80 (64, 91) and 62 (50, 81) postpartum; helpfulness ratings of coaching calls were 85 (58, 98). At 37 weeks gestation, 77% of participants achieved IOM weight gain recommendations compared to 54% in the comparison group.
Conclusions
This study provides evidence for the feasibility and acceptability of a remotely‐delivered behavioural weight control intervention in pregnancy and postpartum.
Keywords: gestational weight gain, postpartum weight loss, pregnancy, remote lifestyle intervention
1. INTRODUCTION
Excessive gestational weight gain (GWG) is a modifiable risk factor for obesity in women. 1 , 2 , 3 As most women seek prenatal care and are motivated to have a healthy baby, the pregnancy and postpartum periods provide opportunities to promote sustainable healthy lifestyle behaviours. 4 The American College of Obstetrics and Gynaecology (ACOG) recommends counselling patients to meet the Institute of Medicine's (IOM) GWG goals as part of “standard of prenatal care”. 5 , 6 However, in a busy obstetrical practice there are multiple barriers to carrying out effective weight counselling. 7
Strong and increasing evidence supports behavioural interventions to limit GWG and improve health behaviours. 8 , 9 , 10 , 11 , 12 , 13 Studies have also shown that postpartum behavioural weight loss interventions after delivery can help women reduce postpartum weight retention (PPWR) and risk for future obesity. 14 , 15 , 16 , 17 Because adults face multiple demands on their time, remote delivery (i.e. phone) of behavioural counselling and integration of such counselling within the prenatal care setting where women receive usual care has the potential to enhance patient engagement and satisfaction and to promote dissemination. In addition, incorporating technologies, such as web‐based platforms and mobile applications for behavioural tracking and intervention delivery, are desirable and effective in younger populations, including reproductive‐age women. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Although remotely‐delivered counselling has been shown to be effective for weight loss in adults, 27 few studies have assessed its feasibility and effectiveness for weight management in pregnant and postnatal women. 28 , 29 , 30 , 31
The aim of this pilot study was to evaluate the feasibility, acceptability and preliminary effectiveness of “Healthy for Two/Healthy for You” (H42/H4U), a remotely‐delivered, behavioural health coaching program to limit GWG and PPWR in pregnant and postpartum women engaged in prenatal care. The primary outcomes were feasibility (coach call and learning activity completion, self‐monitoring frequency) and acceptability (perceived helpfulness) of the intervention. To inform the design of future studies, the effectiveness of the H42/H4U program on reducing GWG and 3‐month PPWR compared to a health education comparison group was also evaluated.
2. METHODS
This study was a two‐arm, randomized controlled trial comparing the H42/H4U intervention to a health education comparison group. The trial was registered in clinicaltrials.gov ‐ NCT03551054.
Participants were recruited from one of two hospital‐based obstetric practices in Baltimore, MD, 2016–2017. Obstetric nurses screened all new obstetric patients for eligibility during routine obstetric nurse visits. Interested participants completed an oral consent enabling further assessment of medical eligibility by study physicians. Upon meeting eligibility criteria, participants provided written informed consent.
The study included women, ≥ 18 years, 11–16 gestational weeks of a singleton pregnancy, and BMI ≥ 18.5 kg/m2 at the time of screening. To be eligible, potential participants had to be a patient at one of the two participating obstetric practices, be fluent in English and have regular access to the Internet and a smartphone they were willing to use for the study. Exclusions were: pre‐pregnancy diabetes (type I or II); poorly controlled blood pressure (>160/100 mmHg); prior history of severe preeclampsia or pre‐term birth (before 32 weeks gestation); prior or current diagnosis of incompetent cervix; a serious medical condition hindering use of IOM's recommendations for weight gain in pregnancy; and/or inability or willingness to track food intake for ≥ 3 days (run‐in).
2.1. Randomization and study groups
Randomization, in equal allocations with block size of 4, was stratified according to baseline BMI status (normal weight versus overweight/obese) with the use of a web‐based program. After meeting eligibility criteria and providing written informed consent, participants were randomized to one of the two intervention arms. Those in the H42/H4U intervention arm were trained to use a mobile phone application (Lose It!) to track their weight, dietary intake, and exercise minutes. Those in the health education comparison arm received a single, 45‐minute health education session led by study staff focused on basic nutrition; low‐cost, healthy shopping; and food and general prenatal safety.
2.2. Intervention group: H42/H4U
H42/H4U is a remotely‐delivered health coaching program designed to limit GWG (H42) and reduce PPWR (H4U). The intervention was adapted from previously published behavioural weight loss trials that employed remote approaches 27 (e.g., telephonic coaching, technology tools for tracking) to facilitate behaviour change strategies (e.g., goal setting, self‐monitoring) and existing weight management interventions for pregnant and postpartum women. 8 , 13
Participants were encouraged to meet IOM pregnancy weight gain guidelines based on pre‐pregnancy BMI (normal weight: 25–35 lb; overweight: 15–25 lb; obese: 11–20 lb) and to return to their pre‐pregnancy weight postpartum. The postpartum program lasted 12 weeks; women classified as overweight or obese pre‐pregnancy were encouraged to lose an additional 5% within the first postpartum year. Daily calorie targets during pregnancy (no less than 1800 kcal) were assigned based on age, height, and pre‐pregnancy weight as recommended by the IOM, adjusting for pre‐pregnancy BMI, as recommended by 2008 Evidence of Analysis for GDM. 6 , 32 , 33 Postpartum calorie targets were based on weight at 14 days postpartum, weight goal (loss or maintenance) and breastfeeding status (breastfeeding defined as four or more breast/pump feedings per day). Based on current guidelines from ACOG, 34 unless otherwise directed by their OB, participants were encouraged to gradually increase to at least 150 minutes of moderate intensity aerobic exercise per week (i.e., 30 minutes on at least 5 days/week) during pregnancy and the postpartum period.
Weight, dietary and physical activity objectives were promoted through: i) patient‐centered coaching phone calls, ii) learning materials based on the COACH model (Commit, Omit, Add, Communicate, Honour your wellness), and iii) self‐monitoring of weight, calories and exercise minutes (Table 1). Health coaching calls were approximately 20–30 minutes and occurred weekly during the first 12 weeks of the program and biweekly thereafter, until 3 months postpartum. Coaching calls were scheduled in advance, with at least 3 additional attempts made by the coach to reschedule missed calls. Coaches held Master's degrees and had strong backgrounds in behavioural health coaching, nutrition, and/or maternal health. Training in motivational interviewing (MI) was provided at the onset of the trial by a member of the MI Network of Trainers. An intervention oversight team listened to audio‐recordings and provided feedback to Coaches bi‐monthly, monthly, then quarterly, to assure fidelity to the behavioural protocol and to promote a patient‐centered approach.
TABLE 1.
Intervention Description
| Duration of intervention |
~14 weeks gestation to 12 weeks postpartum (~38 weeks)* Pregnancy: ~26 weeks* Postpartum: 12 Weeks |
|---|---|
| Coach Calls |
~20–30 minutes per call Weekly for first 12 weeks of program (12 calls) Every other week until delivery (~7 calls)* and for 12 weeks after delivery (6 calls) Patient‐centered approach with individualized goal setting |
| Learning Activities |
Learning activities with diet/physical activity/behaviour change strategies and wellness (e.g. sleep, stress, mood) topics following the COACH framework; Provided paper and electronic copies prior to Coach Calls |
| COACH Framework |
Commit to coach calls, tracking weight, calories and exercise, attending prenatal visits, and completing learning activities; Omit/Limit high‐fat, high‐sugar, processed foods/beverages; Add/Substitute fruits/vegetables, healthy fats, fibre, etc., and exercise; Communicate with your Coach, health care team, family, friends, co‐workers to increase support; H o363200864nouryour wellness (mood, stress and sleep management) |
| Smart‐phone based self‐monitoring |
Weekly weighing; Daily self‐monitoring of caloric intake and moderate intensity exercise |
The number of calls during pregnancy and postpartum assumes that all women began the intervention at 14 weeks pregnancy and delivered at 40 weeks. However, given that: 1) delivery dates were variable (36–40 weeks), and 2) some women faced medical complications at delivery that delayed onset of postpartum calls, the “expected” number of calls during the biweekly phase of pregnancy and postpartum could vary slightly.
Study investigators developed learning materials that covered a range of dietary/eating, physical activity, interpersonal, wellness, and pregnancy topics using the COACH framework, and provided both electronic and hard copies to participants. Although no specific diet was endorsed, the DASH diet 35 was provided as an example of a healthy eating plan, and the general dietary approach was to omit/limit high‐fat, high‐sugar foods and beverages and to add/substitute fruits and vegetables, low‐fat, high fibre foods and lean proteins. Safe and regular moderate exercise (150 or more mins/week) and communication with friends, family and providers to improve social support were also promoted, as were components of wellness, including improving sleep, stress and mood management.
Participants were asked to weigh themselves at least weekly and to enter their weight on a readily available, free smartphone application (Lose It!), where they were also asked to enter their daily dietary intake and moderate (or higher) exercise minutes. Participants were given the option to enable app‐based notifications within the Lose It! app; however, no study‐initiated automated prompts were provided. Coaches used a web‐interface called “Ascend” for the Lose It! Mobile App to view tracking data (https://ascendapp.com); providing feedback on tracking information was a key component of intervention calls with participants.
2.3. Health education group
Women randomized to the health education comparison group received a single, in‐person session at the time of randomization lasting approximately 30 minutes. Participants were provided educational materials on foods to avoid during pregnancy, increasing fruits and vegetables, and basic pregnancy “do's and don'ts” (e.g., do see your doctor regularly; do not smoke tobacco or use illegal drugs). Participants were given the opportunity to ask questions and share their thoughts, however, no self‐monitoring recommendations or individualized weight gain guidelines outside what their obstetrician or clinic staff discussed were provided.
2.4. Data collection
Height (measured) and demographic and behavioural information (electronically self‐reported) were assessed at baseline. Weights were measured by trained study staff during in‐person clinical visits at 3 time‐points: baseline enrollment (randomization), 37 weeks gestation (delivery), and 12 weeks postpartum. In cases where participants delivered early (n = 2; 1 in H42/H4U, n =1 in Health Education) or were unable to make the 37‐week (n = 1 in H42/H4U) or postpartum clinic visit (n = 1 in H42/H4U), weights were abstracted from the electronic medical record (total of 4 weights abstracted).
Participant demographics, including race, age, income, education and employment status were obtained at baseline using a self‐report questionnaire.
Baseline behavioural characteristics, including dietary (sugar‐sweetened beverage, fast‐food, and fruit/vegetable intake) and exercise (frequency of days/week) behaviours were assessed using the National Cancer Institutes' Healthy Eating Index Screeners and the Godin Leisure‐Time Exercise Questionnaire, respectively. 36 , 37
Standing height (millimetres) was an average of two measurements taken at baseline, without shoes, using a calibrated stadiometer.
Body weight (kilograms) was obtained using a digital calibrated scale with participants in light clothing, without shoes and jewellery. Scale calibration was checked weekly.
2.5. Feasibility and acceptability outcomes
Intervention feasibility was measured throughout the intervention using web‐generated, participant‐ and Coach‐reported data in the H42/H4U group. Indicators of feasibility included dose (call completion) and engagement (learning activity completion and frequency of tracking of weight, calories and exercise). Frequency of tracking was defined as: 1) number of weeks weight was tracked at least once, 2) number of days > 2 meals were logged (calories), and 3) number of days > 10 minutes of “moderate” or greater exercise was logged.
Intervention acceptability was measured at follow‐up using sliding visual analogue scales (0–100) that assessed perceived helpfulness (“not at all helpful” to “extremely helpful”) of program components (e.g., general attainment of health goals during pregnancy and postpartum, health coaching, learning activities, and smartphone tracking). Likelihood to refer family or friends and enrol in the program again (“not at all likely” to “highly likely”) was also assessed.
2.6. Preliminary effectiveness outcomes
The study was not powered to detect differences in weight gain by randomized group. Still, we report preliminary, clinic‐derived, research staff‐measured weight outcomes. Gestational weight gain was defined as the difference between 37‐week and pre‐pregnancy weight. Postpartum weight retention was defined as the difference between weight at 3‐months postpartum and pre‐pregnancy weight. Smaller positive differences corresponded to less weight gain during pregnancy and less postpartum weight retention.
3. STATISTICAL ANALYSES
Participant characteristics (Table 2) are reported as counts and percentages (categorical variables) and as means and standard deviations (continuous variables). Intervention feasibility and acceptability data are presented as medians and percentiles (25th, 75th) for the H42/H4U group (Table 3). Secondary outcomes of GWG and PPWR were analysed according to the intention‐to‐treat principle using a quantile regression to estimate difference in median weight change, and Fisher's exact test to assess difference in % reaching IOM guidelines during pregnancy, between groups. Analyses were conducted using STATA version 14.
TABLE 2.
Baseline characteristics of health coaching and health education group participants
| Baseline variable | Health Coaching (n = 13) | Health Education (n = 13) | |
|---|---|---|---|
| Age, mean (SD) | |||
| 32.7 (4.3) | 30.6 (2.5) | ||
| Baseline BMI, mean (SD) | |||
| Baseline BMI groups, n (%) | 25.5 (4.0) | 27.8 (9.7) | |
| Normal weight (18.5–24.9) | 7 (54%) | 6 (46%) | |
| Overweight (25.0–29.9) | 4 (31%) | 5 (38%) | |
| Obese (> 30) | 2 (15%) | 2 (15%) | |
| Race, n (%) | |||
| Caucasian | 11 (85%) | 4 (31%) | |
| African‐American | 2 (15%) | 4 (31%) | |
| Asian | 0 | 3 (23%) | |
| Multiethnic/mixed | 0 | 2 (15%) | |
| Highest education, n (%) | |||
| Some high school (9‐11 th grade) | 1 (8%) | 0 | |
| College (1–3 years) | 0 | 2 (15%) | |
| College graduate | 5 (38%) | 3 (23%) | |
| Post‐graduate | 7 (54%) | 8 (62%) | |
| Employment, n (%) | |||
| Full‐time | 12 (92%) | 12 (92%) | |
| Homemaker | 0 | 1 (8%) | |
| Unemployed | 1 (8%) | 0 | |
| Fast Food meal/snack, n (%) | |||
| Never | 6 (46%) | 5 (38%) | |
| 1–3 per month | 4 (31%) | 5 (38%) | |
| 4–6 per month | 0 | 1 (8%) | |
| 1–3 per week | 3 (23%) | 2 (15%) | |
| Sugar‐sweetened drinks, n (%) | |||
| Never or < 1 per week | 10 (77%) | 8 (62%) | |
| 1–2 per week | 2 (15%) | 3 (23%) | |
| 1 per day | 0 | 2 (15%) | |
| > 2 per day | 1 (8%) | 0 | |
| >15 min exercise, mean (SD) | |||
| Strenuous per week | 1.5 (0.9) | 1.5 (1.5) | |
| Moderate to strenuous per week | 3.6 (1.7) | 4.2 (2.9) | |
| Moderate per week | 2.2 (1.2) | 2.6 (1.6) | |
| Mild per week | 3.1 (2.1) | 3.3 (2.1) | |
TABLE 3.
Intervention Feasibility and Acceptability Data for H42/H42U Group (n = 11*)
| Feasibility | ||
|---|---|---|
| Coaching Calls | ||
| Pregnancy ** | Postpartum *** | |
| Recommended | 19 | 6 |
| Median | 18 | 6 |
| Quartile 1 | 17 | 5 |
| Quartile 3 | 19 | 6 |
| Learning Activities | ||
| Recommended | 19 | 6 |
| Median | 16 | 5 |
| Quartile 1 | 14 | 4 |
| Quartile 3 | 17 | 6 |
| Tracking **** | |||
| Weight (weeks) | Calories (days) | Exercise (days) | |
| Recommended | 38 | 266 | 266 |
| Median | 36 | 154 | 72 |
| Quartile 1 | 35 | 146 | 20 |
| Quartile 3 | 37 | 216 | 98 |
| Acceptability (0 = not helpful, 100 = very helpful) Median (25th, 75th percentile) | |
| Helpfulness of … | |
| H42/H4U program for reaching pregnancy health goals | 80 (64, 91) |
| H42/H4U program for reaching postpartum health goals | 62 (50, 81) |
| Telephone calls | 85 (58, 98) |
| Learning Activities | 64 (33, 73) |
| Tracking weekly weight | 76 (58, 99) |
| Tracking type of food and daily calories | 77 (63, 97) |
| Tracking daily exercise | 74 (51, 79) |
| Individualized feedback on tracking behaviours | 82 (62, 100) |
| Ease of scheduling coaching calls | 96 (84, 100) |
| Ease of using app to track | 99 (73, 100) |
| Likeliness to … | |
| Refer a friend or family member | 83 (51, 92) |
| Join program again (if you become pregnant) | 73 (49, 100) |
Total number of women that received the intervention (See Figure 1).
The expected number of pregnancy calls and learning activities was modified based on actual delivery date. 4 women delivered 1–2 weeks early.
The expected number of postpartum calls and learning activities was modified based on medical clearance to resume calls 14 days postpartum. 1 woman was unable to complete her first call due to labor complications.
The expected number of weeks and days tracked equals total study duration, similarly modified based on actual delivery date and onset of postpartum calls.
3.1. Human subject protection
The study was approved by the Johns Hopkins' Institutional Review Board.
4. RESULTS
Figure 1 shows the flow of participants through the study. Of the 659 participants presenting for an initial obstetric nurse visit over 18 months, 483 did not meet initial eligibility criteria, most commonly due to being > 16 weeks gestation (n = 215) or lack of interest (n = 201). Of the remaining 176, 79 were unable to be reached for further screening, 44 declined to participate or were no longer eligible based on gestational age, and 53 orally consented for further eligibility assessment, half of whom did not make it to randomization. Twenty‐six women provided written consent to participate and were randomized to H42/H4U (n = 13) or the health education comparison group (n = 13).
FIGURE 1.
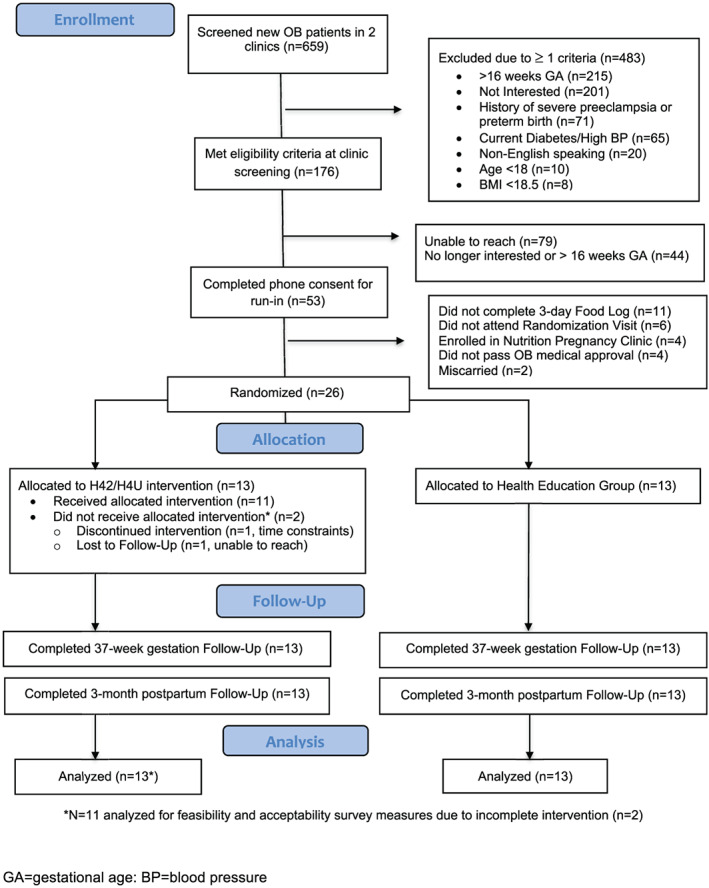
Legend. Participant Flow/Recruitment
Baseline characteristics by group are included in Table 2. Overall, participants had a mean age of 31.7 years (SD = 3.6), were racially diverse (White = 57.7%, Black = 23.1%, Asian = 11.5% and Multiethnic = 7.7%), were between 11–16 weeks gestation (mean = 13.9, SD = 1.3) at time of enrollment, and had a mean baseline BMI of 26.7 kg/m2 (SD = 7.3; 50%, 35%, 15% were normal, overweight and obese, respectively). In regards to baseline dietary and physical activity behaviours, 69.2% of participants drank sugar‐sweetened beverages “never or <1 per week”, 77.0% ate fast food “never or < 1‐3 times per month”, and participants reported exercising at a moderate to vigorous pace for 15 minute bouts or more an average of 2.6 times per week.
Participants in the H42/H4U group were more likely to be white than women in the health education group (n = 11, 85%; p = .024). No other differences in baseline demographic or behavioural variables were detected between participants in the H42/H4U versus health education group.
4.1. Feasibility and acceptability
Table 3 shows the actual and recommended rates of participation in H42/H4U. The median number of completed phone calls was 18 (Q1:17, Q3: 19) out of 19 possible calls in the pregnancy phase of the intervention and 6 (Q1: 5, Q3: 6) out of 6 possible calls postpartum. H42/H4U participants completed a median of 16 (Q1: 14, Q3: 17) of 19 possible learning activities during pregnancy and 5 (Q1: 4, Q3: 6) of 6 possible learning activities postpartum. Throughout the entire intervention (~14 weeks gestation through 12 weeks postpartum, ~38 weeks, ~266 days), H42/H4U participants tracked their weight a median of 36 weeks (Q1: 35, Q3: 37) out of the 38 possible weekly entries; they tracked calories and exercise a median of 154 days (Q1: 146, Q3: 216) and 72 days (Q1: 20, Q3: 98) out of the 266 possible daily entries, respectively.
As shown in Table 3, the median “intervention acceptability” ratings for each of the individual intervention components ranged from 62–99 (on a 0–100 scale, higher indicates more helpful). The components rated most acceptable were ease of using the app to track (median = 99), ease of scheduling coaching calls (median = 96), and overall helpfulness of the coaching calls (median = 85). H42/H4U participants found the program least helpful at helping them reach their postpartum goals (median = 62). Half of the participants rated the likeliness to refer a friend or family member to the program at 83 (on a 0–100 scale, higher indicates more likely) or higher. The median rating for likeliness to join the program again (if pregnant) was 73.
4.2. Weight outcomes
The median change in weight from baseline to 37 weeks gestation was 9.4 kg in the H42/H4U group and 13.0 kg in the health education comparison group (p = 0.16). At 12 weeks postpartum, the median change in weight from baseline was 0.2 kg in the H42/H4U group and 1.4 kg in the comparison group (p = .44; See Figure 2). Corresponding means and standard deviations are as follows: mean change in weight from baseline to 37 weeks gestation was: 11.4 kg (SD = 4.5) in the H42/H4U group and 12.0 kg (SD = 4.5) in the health education comparison group. At 12 weeks postpartum, mean change in weight from baseline was 0.9 kg (SD = 4.5) in the H42/H4U group and 2.6 kg (SD = 3.7) in the comparison group. The percentage of participants who met IOM weight gain guidelines at 37 weeks of pregnancy was 77% (n = 10; 7 normal weight, 2 overweight, 1 obese) in the H42/H4U group and 54% (n = 7; 5 normal weight, 1 overweight, 1 obese) in the health education group (p = 0.41).
FIGURE 2.
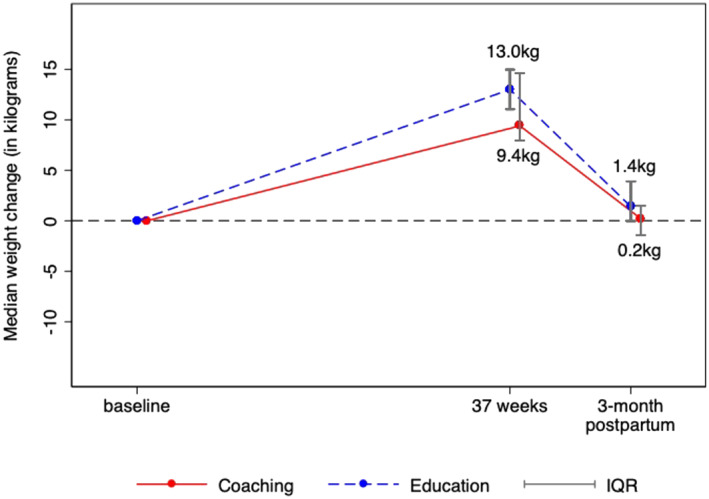
Legend. Median weight change during pregnancy and 12 weeks postpartum for H42/H4U and health education groups
5. DISCUSSION
In this pilot study that enrolled normal, overweight and obese pregnant women, a remotely‐delivered, behavioural health coaching intervention to limit GWG and PPWR was both feasible and acceptable. Although we did not design this pilot trial to have sufficient statistical power to detect between‐group weight differences, there was a tendency toward reduced GWG and PPWR in those assigned to H42/H4U. Participants were screened and enrolled in a real‐world prenatal care setting; therefore, the study also provided useful information about recruitment yields, attrition rates and future implementation into care.
Participants were highly engaged in the H42/H4U intervention both during and after pregnancy. Most women (82%; n = 9) completed at least 80% of their recommended pregnancy calls and many (55%; n = 6) completed at least 90% of these calls. In the postpartum phase of the H42/H4U intervention, most women (73%; n = 8) completed at least 80% of their recommended calls and almost half of the women (n = 5) had 100% call completion. The patient‐centered nature of the H42/H4U program, assignment to one continuous health coach throughout the program, and ease of scheduling calls may have contributed to engagement success, as these features were all highly rated by participants. Another important and novel component of H42/H4U was starting coaching early in pregnancy and continuing through 12 weeks after delivery. Few prior studies have included both pregnancy and postpartum intervention phases. 8 , 38 , 39 , 40 A recently published systematic review of 14 RCTs of lifestyle interventions in pregnancy (5 extended into the postpartum period) showed significantly less PPWR in the intervention compared to control groups, but without an effect in the 5 studies that only included women with BMI ≥ 25 kg/m. 2 , 40 Interventions that begin after delivery may miss out on teachable moments during pregnancy and result in lower engagement rates, since new mothers are less likely to focus on their own health. 4
This study also achieved high levels of participant engagement through completion of learning activities and remote‐tracking of health behaviours. Half of participants completed at least 80% of learning activities (16/19 and 5/6 in pregnancy and postpartum, respectively), an important finding since these materials contained strategies for reaching the core behavioural targets of H42/H4U. Throughout the program, 82% (n = 9) of H42/H4U participants tracked their weight > 90% of the recommended weeks and tracked their calories > 60% of the recommended days, a promising finding given the well‐documented value of self‐monitoring in weight management studies. 41 , 42 Positive overall feedback on self‐monitoring, including perceived helpfulness and ease of using the tracking app may have contributed to these high tracking rates. The finding that 73% (n = 8) of participants tracked their exercise less than 30% of the recommended days, 45% (n = 5) of whom tracked 10% of these days or less, may be a reflection of the barriers that pregnant women face (e.g., physical discomfort, fatigue, soreness; body image issues; concern about injury). 43 It also reflects a challenge in the measurement of exercise logging, as participants are guided to exercise for at least 150 minutes or more of moderate exercise per week, which is equivalent to exercising on most days of the week (~5). Without objective measures of exercise, it cannot be determined whether the low rates of logging are a proxy for infrequent exercise or missed logging. Future studies should include accelerometers or other wearable devices to help clarify this finding. 44 Moreover, programs that provide personalized automated feedback and tracking reminders/notifications may increase overall tracking success. 45
Although this feasibility and acceptability study was not powered to detect a between group difference in GWG but rather to inform future study design, we found that 77% of participants in the H42/H4U intervention met IOM weight gain recommendations during pregnancy vs. 54% in the health education group. Consistent with other studies that included women with all pre‐pregnancy BMI's, we showed a greater proportion of participants in a lifestyle intervention (addressing both diet and exercise) gaining within IOM recommendations compared to controls. 46 , 47 , 48 Furthermore, evidence from the pooled analysis from the LIFE‐Moms clinical trial consortium of 7 independent trials totaling 1,150 diverse overweight or obese pregnant women revealed that excess GWG was significantly lower in the intervention group compared to standard care (61.8% vs. 75.0%; p < 0.001). 8 A feature that distinguished the current study from those in the pooled study was that most of the interventions in the pooled analysis included in‐person counselling with limited online and mobile technologies as compared to the exclusively remote delivery of the H42/H4U program. 8
Remotely delivered programs may reduce barriers to participant access and promote sustainability and implementation across multiple practices to ultimately enhance participant engagement. 49 Increasingly, health care systems, health insurers and employers are utilizing a wide range of “population health” approaches to improve health outcomes for the communities they serve, often through care management and health coaching. 50 , 51 , 52 A major strength of H42/H4U is its interface with prenatal care through the initial patient enrollment process and its remote delivery model. In our prior study evaluating the role of health care providers in a weight loss intervention, busy providers appreciated: 1) the remote delivery of the program, 2) having the coach (and not the provider) as the “interventionist” and 3) having the availability of progress reports for review. 53 Although the use of remote intervention delivery is promising, the degree to which innovative technology (e.g., weight graphs, tailored text messages, automated reminders, etc.) can enhance or replace human delivered coaching is unknown, and minimally effective coaching doses have yet to be identified. For example, some studies have demonstrated benefits of remotely‐delivered interventions with less intensive coaching frequency than offered in H42/H4U, 31 particularly for study completers, 29 while studies with call frequencies more comparable to, or more intensive than, H42/H4U have yielded mixed results. 30 , 54 Future research should consider the utility of adaptable intervention designs in producing clinically meaningful, yet scalable results.
There are several limitations of this study. First, the sample size was small, precluding firm conclusions beyond the study goal of feasibility and acceptability. The greatest barrier to enrollment was women presenting with later gestational age, highlighting the need to broaden gestational age eligibility and allow flexible start times for the intervention. Despite these challenges, 37‐week and 12‐week postpartum weights were collected in the prenatal clinic for 76.9% (n = 10) and 92.3% (n = 12) of participants, respectively, suggesting that working within a prenatal clinic is a successful retention strategy. Future studies might consider additional strategies, such as working with paediatrician and primary care clinics, since mothers tend to focus their postpartum energy on paediatric visits and often transition their care to primary care providers. Next, intervention findings are most generalizable to white, well‐educated, employed women with generally healthy baseline behaviours. Although we used an established method for adapting health education materials for lower health literacy populations, 55 until further testing we cannot assess the degree to which H42/H4U is appropriate for participants with lower literacy. Future recruitment of those with lower education and socioeconomic status will allow for more generalizable results related to tracking and call completion rates, accounting for issues such as data plan loss, etc.
In conclusion, this feasibility and acceptability study demonstrates that a remotely‐delivered behavioural health coaching intervention, recruiting within the prenatal care setting, is both acceptable to patients and providers, and feasible to carry out to promote weight management during pregnancy and the postpartum period. Results are promising, particularly because of the need for implementing programs like H42/H4U into the prenatal care setting where they can have the greatest impact on patient care. Further research will examine the effectiveness of H42/H4U when it is fully integrated as a clinical program, with provider involvement and electronic health record tools, as well as the longer‐term effects of the intervention on maternal and offspring obesity and related health outcomes (e.g., incident diabetes and hypertension, and ultimately cardiovascular disease).
FUNDING
This study was funded in part by a Pilot and Feasibility Grant from the NIDDK‐funded Mid‐Atlantic Nutrition Obesity Research Center (5P30DK072488) and by NIH/NHLBI (5R03HL136793).
ACKNOWLEDGEMENTS
The authors would like to thank Jennifer Thrift, MS, CHES, Manager of Health Promotion and Wellness Unit Care, Care Management Department, from Johns Hopkins HealthCare, LLC, who supported and advised on intervention development and coaching delivery, and Ann Israel, MA, FACCE, NBC‐HWC, who served as a health coach, as part of her role at Johns Hopkins HealthCare, LLC. We would also like to thank Christine McKinney, MS, RDN, CDE, and Gerald Jerome, PhD, Associate Professor of Kinesiology at Towson University, who served as advisors about diet and exercise in pregnancy, respectively.
Coughlin JW, Martin LM, Henderson J, et al. Feasibility and acceptability of a remotely‐delivered behavioural health coaching intervention to limit gestational weight gain. Obes Sci Pract. 2020;6:484–493. 10.1002/osp4.438
REFERENCE
- 1. Goldstein RF, Abell SK, Ranasinha S, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta‐analysis. JAMA. 2017;317:2207‐2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kominiarek MA, Peaceman AM. Gestational weight gain. Am J Obstet Gynecol. 2017;217:642‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilmore LA, Klempel‐Donchenko M, Redman LM. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Semin Perinatol. 2015;39:296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol. 2010;202(2):135.e1‐135.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ACOG . Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013;121:210‐212. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen KM, Yaktine AL. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM pregnancy weight guidelines. Weight Gain During Pregnancy: Reexamining The Guidelines. Washington (DC): National Academies Press (US); 2009:2‐9. [PubMed] [Google Scholar]
- 7. Washington Cole KO, Gudzune KA, Bleich SN, et al. Influence of the 5A's Counseling Strategy on Weight Gain During Pregnancy: An Observational Study. J Womens Health (Larchmt). 2017;26:1123‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peaceman AM, Clifton RG, Phelan S, et al. Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE‐Moms Prospective Meta‐Analysis. Obesity (Silver Spring). 2018;26:1396‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hedderson MM, Brown SD, Ehrlich SF, et al. A Tailored Letter Based on Electronic Health Record Data Improves Gestational Weight Gain Among Women With Gestational Diabetes Mellitus: The Gestational Diabetes' Effects on Moms (GEM) Cluster‐Randomized Controlled Trial. Diabetes Care. 2018;41:1370‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cahill AG, Haire‐Joshu D, Cade WT, et al. Weight Control Program and Gestational Weight Gain in Disadvantaged Women with Overweight or Obesity: A Randomized Clinical Trial. Obesity (Silver Spring). 2018;26:485‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skouteris H, Hartley‐Clark L, McCabe M, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obes Rev. 2010;11:757‐768. [DOI] [PubMed] [Google Scholar]
- 12. Flynn AC, Dalrymple K, Barr S, et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016;74:312‐328. [DOI] [PubMed] [Google Scholar]
- 13. Clifton RG, Evans M, Cahill AG, et al. Design of lifestyle intervention trials to prevent excessive gestational weight gain in women with overweight or obesity. Obesity (Silver Spring). 2016;24:305‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicodemus NA Jr. Prevention of Excessive Gestational Weight Gain and Postpartum Weight Retention. Curr Obes Rep. 2018;7:105‐111. [DOI] [PubMed] [Google Scholar]
- 15. Ferrara A, Hedderson MM, Brown SD, et al. The Comparative Effectiveness of Diabetes Prevention Strategies to Reduce Postpartum Weight Retention in Women With Gestational Diabetes Mellitus: The Gestational Diabetes' Effects on Moms (GEM) Cluster Randomized Controlled Trial. Diabetes Care. 2016;39:65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes VA, Draffin CR, Patterson CC, et al. Postnatal Lifestyle Intervention for Overweight Women With Previous Gestational Diabetes: A Randomized Controlled Trial. J Clin Endocrinol Metab. 2018;103:2478‐2487. [DOI] [PubMed] [Google Scholar]
- 17. van der Pligt P, Willcox J, Hesketh KD, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013;14:792‐805. [DOI] [PubMed] [Google Scholar]
- 18. Daly LM, Horey D, Middleton PF, Boyle FM, Flenady V. The effect of mobile app interventions on influencing healthy maternal behavior and improving perinatal health outcomes: systematic review. JMIR mHealth and uHealth. 2018;6(8):e10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chilukuri N, West M, Henderson JL. Information and communication technology use among low‐income pregnant and postpartum women by race and ethnicity: a cross‐sectional study. J Med Internet Res. 2015;17(7):e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Acquavita SP, Krummel DA, Talks A, Cobb A, McClure E. Assessing the Digital Divide Among Low‐Income Perinatal Women: Opportunities for Provision of Health Information and Counseling. Telemed J E Health. 2019;25:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayman M, Reaburn P, Browne M, Vandelanotte C, Alley S, Short CE. Feasibility, acceptability and efficacy of a web‐based computer‐tailored physical activity intervention for pregnant women ‐ the Fit4Two randomised controlled trial. BMC Pregnancy Childbirth. 2017;17:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham ML, Strawderman MS, Demment M, Olson CM. Does usage of an eHealth intervention reduce the risk of excessive gestational weight gain? Secondary analysis from a randomized controlled trial. J Med Internet Res. 2017;19(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silfee VJ, Lopez‐Cepero A, Lemon SC. Adapting a behavioral weight loss intervention for delivery via facebook: a pilot series among low‐income postpartum women. JMIR Form Res. 2018;2(2):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilmore LA, Klempel MC, Martin CK, et al. Personalized Mobile Health Intervention for Health and Weight Loss in Postpartum Women Receiving Women, Infants, and Children Benefit: A Randomized Controlled Pilot Study. J Womens Health (Larchmt). 2017;26:719‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herring SJ, Cruice JF, Bennett GG, Davey A, Foster GD. Using technology to promote postpartum weight loss in urban, low‐income mothers: a pilot randomized controlled trial. J Nutr Educ Behav. 2014;46:610‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willcox JC, Wilkinson SA, Lappas M, et al. A mobile health intervention promoting healthy gestational weight gain for women entering pregnancy at a high body mass index: the txt4two pilot randomised controlled trial. BJOG. 2017;124:1718‐1728. [DOI] [PubMed] [Google Scholar]
- 27. Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365:1959‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Redman LM, Gilmore LA, Breaux J, et al. Effectiveness of SmartMoms, a Novel eHealth Intervention for Management of Gestational Weight Gain: Randomized Controlled Pilot Trial. JMIR Mhealth Uhealth. 2017;5:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phelan S, Phipps MG, Abrams B, et al. Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve‐month outcomes of the Fit for Delivery randomized trial. Am J Clin Nutr. 2014;99:302‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chao AM, Srinivas SK, Studt SK, Diewald LK, Sarwer DB, Allison KC. A Pilot Randomized Controlled Trial of a Technology‐Based Approach for Preventing Excess Weight Gain during Pregnancy among Women with Overweight. Front Nutr. 2017;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: A randomized clinical trial. Obesity (Silver Spring). 2016;24:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Langer O, Yogev Y, Xenakis EM, Brustman L. Overweight and obese in gestational diabetes: the impact on pregnancy outcome. Am J Obstet Gynecol. 2005;192:1768‐1776. [DOI] [PubMed] [Google Scholar]
- 33. Rae A, Bond D, Evans S, North F, Roberman B, Walters B. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Aust N Z J Obstet Gynaecol. 2000;40:416‐422. [DOI] [PubMed] [Google Scholar]
- 34. ACOG . Committee Opinion No. 650: Physical Acitivity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol. 2015;126:e135‐e142. [DOI] [PubMed] [Google Scholar]
- 35. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117‐1124. [DOI] [PubMed] [Google Scholar]
- 36. Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI‐2010. J Acad Nutr Diet. 2013;113:569‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141‐146. [PubMed] [Google Scholar]
- 38. Fernandez ID, Groth SW, Reschke JE, Graham ML, Strawderman M, Olson CM. eMoms: Electronically‐mediated weight interventions for pregnant and postpartum women. Study design and baseline characteristics. Contemp Clin Trials. 2015;43:63‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chasan‐Taber L, Marcus BH, Rosal MC, et al. Proyecto Mama: a lifestyle intervention in overweight and obese Hispanic women: a randomised controlled trial ‐ study protocol. Bmc Pregnancy Childb. 2015;15:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michel S, Raab R, Drabsch T, Gunther J, Stecher L, Hauner H. Do lifestyle interventions during pregnancy have the potential to reduce long‐term postpartum weight retention? A systematic review and meta‐analysis. Obes Rev. 2019;20:527‐542. [DOI] [PubMed] [Google Scholar]
- 41. Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self‐regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563‐1571. [DOI] [PubMed] [Google Scholar]
- 42. Olson CM, Strawderman MS, Graham ML. Association between consistent weight gain tracking and gestational weight gain: Secondary analysis of a randomized trial. Obesity (Silver Spring). 2017;25:1217‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Downs DS, Chasan‐Taber L, Evenson KR, Leiferman J, Yeo S. Physical activity and pregnancy: past and present evidence and future recommendations. Res Q Exerc Sport. 2012;83:485‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta‐analysis of intervention trials. Bjog‐Int J Obstet Gy. 2011;118:278‐284. [DOI] [PubMed] [Google Scholar]
- 45. Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self‐monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring). 2011;19:338‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abdel‐Aziz SB, Hegazy IS, Mohamed DA, Abu El Kasem MMA, Hagag SS. Effect of dietary counseling on preventing excessive weight gain during pregnancy. Public Health. 2018;154:172‐181. [DOI] [PubMed] [Google Scholar]
- 47. Dodd JM, Crowther CA, Robinson JS. Dietary and lifestyle interventions to limit weight gain during pregnancy for obese or overweight women: A systematic review. Acta Obstet Gyn Scan. 2008;87:702‐706. [DOI] [PubMed] [Google Scholar]
- 48. Hill B, Skouteris H, Fuller‐Tyszkiewicz M. Interventions designed to limit gestational weight gain: a systematic review of theory and meta‐analysis of intervention components. Obes Rev. 2013;14:435‐450. [DOI] [PubMed] [Google Scholar]
- 49. Blake‐Lamb T, Boudreau AA, Matathia S, et al. Strengthening integration of clinical and public health systems to prevent maternal‐child obesity in the First 1,000Days: A Collective Impact approach. Contemp Clin Trials. 2018;65:46‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reid N, Bennett W, Coughlin J, Thrift J, Kachur S, Gudzune KA. Evaluating an insurer‐based health coaching program: Impact of program engagement on healthcare utilization and weight loss. Prev Med Rep. 2018;12:343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murphy BM, Schoenman JA, Pirani H. Health insurers promoting employee wellness: strategies, program components and results. Am J Health Promot. 2010;24:e1‐e10. [DOI] [PubMed] [Google Scholar]
- 52. Wolever RQ, Simmons LA, Sforzo GA, et al. A Systematic Review of the Literature on Health and Wellness Coaching: Defining a Key Behavioral intervention in Healthcare. Glob Adv Health Med. 2013;2:38‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bennett WL, Gudzune KA, Appel LJ, Clark JM. Insights from the POWER practice‐based weight loss trial: a focus group study on the PCP's role in weight management. J Gen Intern Med. 2014;29:50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van Horn L, Peaceman A, Kwasny M, et al. Dietary Approaches to Stop Hypertension Diet and Activity to Limit Gestational Weight: Maternal Offspring Metabolics Family Intervention Trial, a Technology Enhanced Randomized Trial. Am J Prev Med. 2018;55:603‐614. [DOI] [PubMed] [Google Scholar]
- 55. Hill‐Briggs F, Schumann KP, Dike O. Five‐step methodology for evaluation and adaptation of print patient health information to meet the < 5th grade readability criterion. Medical Care. 2012;50:294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]


