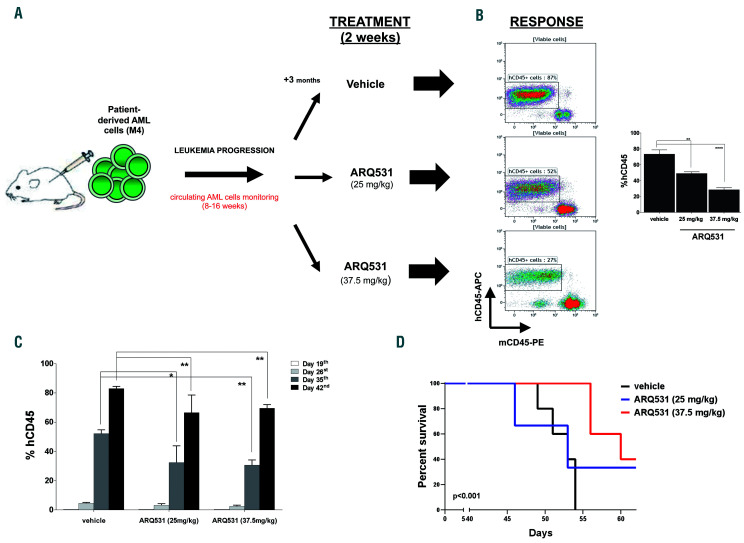
Figure 7.
ARQ531 inhibits tumor growth and extends survival in a patient-derived xenograft mouse model of acute myeloid leukemia. (A) Experimental outline for the analysis of the anti-leukemic activity of ARQ531 against primary human acute myeloid leukemia (AML) cells. A patient-derived xenograft mouse model of human primary AML cells was used to assess the efficacy of ARQ531 against AML cells isolated from patients with AML M4. (B) Representative flow cytometric dot plots representing tumor engraftment evaluated at day 35 after treatment. In the right panel, the histogram represents the percentage of human CD45+ cells in mice. Data are represented as mean ± standard deviation; **P=0.006; ****P<0.001. (C) Circulating human CD45+ cells were measured in peripheral blood by flow cytometry weekly for 2 months. At day 19, a systemic xenograft was confirmed (tumor engraftment) and mice were randomized to receive vehicle control, a low dose of ARQ531 (25 mg/kg) or a high dose of ARQ531 (37.5 mg/kg). The percentage of human leukemic cells in peripheral blood of mice was measured weekly, up to day 42. 0.005<**P<0.008. (D) Kaplan-Meier curve of the patient-derived xenograft AML model following treatment with vehicle, a low dose of ARQ531 (25 mg/kg) or a high dose of ARQ531 (37.5 mg/kg). The higher drug dose led to significantly longer overall survival compared to that of the vehicle-treated, control mice (5 mice/group; P<0.001).