Fig. 4. Intramolecular interactions of polyubiquitin.
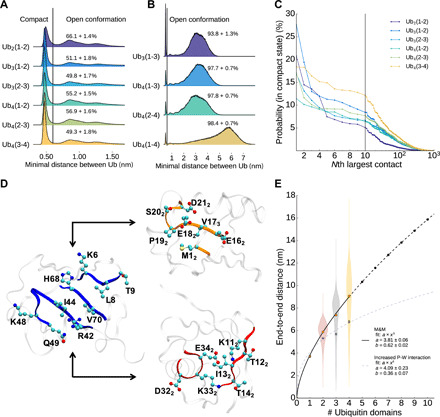
(A) Minimum distance distribution between two neighboring ubiquitin cores (residues 1 to 70, residues 77 to 146, and so forth). Structures with a minimal distance larger than 0.6 nm are defined as open. (B) Minimum distance distribution between two non-neighboring ubiquitin cores. Structures with a minimal distance larger than 0.6 nm are defined as open. (C) Probability of finding contacts between two amino acids of neighboring ubiquitin cores. (D) Interaction surface of two neighboring ubiquitins. Residues from the blue marked surface (first ubiquitin, left) are interacting with residues of the orange marked surface (middle) or red marked surface (right) of the second ubiquitin. (E) Average end-to-end distance of a linear polyubiquitin chain.
