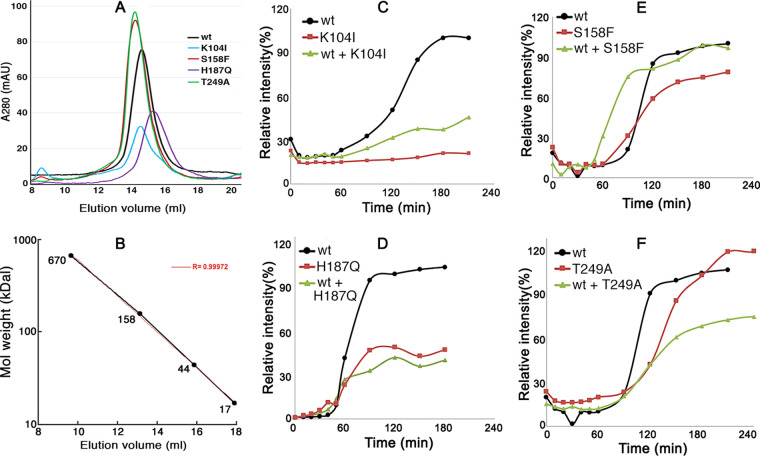
FIG 3.
Ure2 mutations impair amyloid formation. (A) Size exclusion chromatography of purified, full-length Ure2 proteins: wild-type (wt) and CTD mutant (as indicated). (B) Elution of FPLC size standards for the column used in panel A. The expected elution of Ure2 monomers (40.3 kDal) is at 16 ml, dimers at 14.5 ml. (C to F) Kinetics of thioflavin-T binding to purified full-length Ure2 (20 μM). For each plot, the data are normalized to the maximal yield for the wild type, with wild-type Ure2 set at 100%. Results are for wild-type Ure2, indicated mutant Ure2, and mixtures of 10 μM each of wild type and mutant proteins. Due to difficulties in maintaining Ure2 in a soluble form, different preparations of each Ure2 protein had variable lag times and yields of amyloid. For clarity, representative experiments are shown.