Abstract
Takotsubo cardiomyopathy or takotsubo syndrome (TTS) has become a well-known disease not only in Japan but also in the rest of the world. Early reports suggested that TTS is a self-limiting disease with better prognosis than acute coronary syndrome. However, recent data showed that TTS is not a benign disease as compared with acute coronary syndrome. In addition to the apical ballooning, several other types of wall motion abnormalities have been classified as variants of TTS. In particular, right ventricular involvement, or biventricular TTS, is not uncommon and is associated with poor in-hospital as well as long-term outcomes. With respect to the pathophysiology, modulation (desensitization) of the beta-adrenergic receptor is suspected as a possible mechanism for transiently depressed myocardial contraction. Although specific treatments to improve prognosis of TTS are still uncertain, observational data suggest favorable impact of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Finally, in the era of COVID-19, we should pay attention to a variety of cardiovascular conditions related to COVID-19. TTS is one of these conditions that can be triggered by both emotional and physical impact of the COVID-19 pandemic.
Keywords: Takotsubo syndrome, Echocardiography, COVID-19
Introduction
Since the initial reports from Japan [[1], [2], [3], [4]], takotsubo cardiomyopathy or takotsubo syndrome (TTS) has become globally recognized as a unique syndrome mimicking acute coronary syndrome triggered by emotional or physical stress [5]. Initially, emotional stress was considered as essential for TTS and thus TTS was also named as stress-induced cardiomyopathy. However, emotional stress was documented in only 20-39% and physical stress in 35-55% of cases [6]. Interestingly, not only negative emotional stress but also positive emotional stress could be a trigger of TTS, and it has been called “happy heart syndrome” [7]. Natural disasters may cause a variety of cardiovascular diseases, such as acute myocardial infarction [8,9], stroke [10], deep vein thrombosis [10,11], and TTS [12,13]. Currently, the pandemic of COVID-19 affects the health status of people all over the world. SARS-CoV-2 is a newly found corona virus that sometimes causes catastrophic respiratory failure requiring respirator and/or extracorporeal membrane oxygenation. In this review, a current update of TTS in the era of COVID-19 pandemic is summarized.
Pathophysiology and mechanisms of TTS
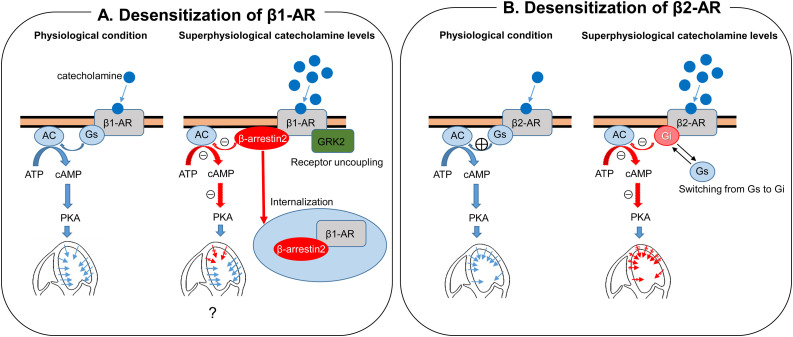
Although catecholamine has been suspected to play some role during the development of TTS, exact mechanisms of TTS are still uncertain. In TTS patients, plasma catecholamine (epinephrine, norepinephrine, and dopamine) levels at presentation were markedly higher than among those with Killip class III myocardial infarction [14]. We further compared catecholamine levels at the aortic root and coronary sinus and demonstrated local release of catecholamine levels (norepinephrine) in TTS [15]. Akashi et al. reported increased iodine-123-meta-iodobenzylguanidine uptake and increased washout ratio in the acute phase of TTS, suggesting the presence of cardiac sympathetic hyperactivity [16]. Paur et al. reported interesting results of an in vivo rat model of TTS. They suggested that high-dose epinephrine can induce direct cardiomyocyte cardiodepression and cardioprotection in a β2-adrenergic receptor (AR)–Gi-dependent manner [17]. At higher concentrations, epinephrine stimulates a negative inotropic effect on myocardial contraction by switching β2-AR coupling from Gs protein to Gi protein [18]. Stimulation of the β2-AR-Gi protein pathway then produces negative inotropic action resulting in akinesis of the involved segments. Location and the extent of wall motion abnormalities in TTS may be explained by the distribution of the β2-AR [18]. More recently, we demonstrated in vivo evidence of β-AR alteration [19]. Left ventricular biopsy samples from patients with TTS demonstrated more abundantly expressed G protein coupled receptor kinase 2 (GRK2) and β-arrestin2, both of which are known to desensitize β-AR, than in samples from dilated cardiomyopathy. Desensitization of β1-AR causes decreased left ventricular contraction of the involved segments. In cases with apical TTS, apical segments may be more involved, although exact mechanisms of the segment specific changes in desensitization of the β1-AR are unclear. Desensitization of β1-AR together with switching of the coupling G protein from Gs to Gi in β2-AR could explain apical involvement because of its apical dominant distribution [[17], [18], [19]] (Fig. 1 ).
Fig. 1.
Proposed mechanisms of wall motion abnormalities in patients with takotsubo syndrome. (A) With superphysiological catecholamine levels, β-arrestin2 internalization the β1-AR and GRK2 induces β1-AR uncoupling, resulting in desensitization and suppression of myocardial contraction. Adapted from Nakano et al. [19]. (B With superphysiological catecholamine levels, stimulation of β2-AR suppress myocardia contraction by switching Gs protein to Gi protein. Adapted from Paur et al. [17].
New classification and diagnostic criteria of TTS
Originally, only those who presented left ventricular apical ballooning or takotsubo-like wall motion were diagnosed as having takotsubo cardiomyopathy or TTS, because only left ventriculography was used to assess wall motion abnormality [5]. As multimodality images have become available [20], we now recognize that apical ballooning was only a part of TTS. A recently published consensus document of multimodality images on TTS well described the role of multimodality images in TTS [20]. There is no doubt that echocardiography plays a pivotal role in the assessment of TTS [6]. Mayo criteria [21] and InterTAK criteria (Table 1 ) [22,23] are well recognized diagnostic criteria. Importantly, InterTAK criteria describe that the presence of pheochromocytoma [1] and significant coronary artery disease (CAD) [24] are not contradictory to the diagnosis of TTS. Because clinical presentation of TTS mimics acute coronary syndrome, the differential diagnosis between the two syndromes is challenging. To differentiate TTS and acute coronary syndrome, the InterTAK Diagnostic Score has been proposed. The InterTAK Diagnostic Score is formed using seven variables, and each was assigned a score value: female sex 25, emotional trigger 24, physical trigger 13, absence of ST-segment depression (except in lead aVR) 12, psychiatric disorders 11, neurologic disorders 9, and QTc prolongation 6 points (Table 2 ). When patients with a score of ≥50 were diagnosed as having TTS, nearly 95% of TTS patients were correctly diagnosed [25].
Table 1.
InterTAK diagnostic criteria.
| International Takotsubo Diagnostic Criteria (InterTAK Diagnostic Criteria) | |
|---|---|
| 1. | Patients show transient left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities. Right ventricular involvement can be present. Besides these regional wall motion patterns, transitions between all types can exist. The regional wall motion abnormality usually extends beyond a single epicardial vascular distribution; however, rare cases can exist where the regional wall motion abnormality is present in the subtended myocardial territory of a single coronary artery (focal takotsubo syndrome). |
| 2. | An emotional, physical, or combined trigger can precede the takotsubo syndrome event, but this is not obligatory. |
| 3. | Neurologic disorders (e.g. subarachnoid hemorrhage, stroke/transient ischemic attack, or seizures) as well as pheochromocytoma may serve as triggers for takotsubo syndrome. |
| 4. | New electrocardiographic (ECG) abnormalities are present (ST-segment elevation, ST-segment depression, T-wave inversion, and QTc prolongation); however, rare cases exist without any ECG changes. |
| 5. | Levels of cardiac biomarkers (troponin and creatine kinase) are moderately elevated in most cases; significant elevation of brain natriuretic peptide is common. |
| 6. | Significant coronary artery disease is not a contradiction in takotsubo syndrome. |
| 7. | Patients have no evidence of infectious myocarditis. |
| 8. | Postmenopausal women are predominantly affected. |
Adapted from Ghadri et al. [22].
Table 2.
InterTAK Diagnostic Score.
| Score | OR (95%CI) | p | |
|---|---|---|---|
| Female | 25 | 68 (29.0−163.7) | <0.001 |
| Emotional trigger | 24 | 65 (20.3−205.8) | <0.001 |
| Physical trigger | 13 | 8.7 (4.6−17.3) | <0.001 |
| Absence of ST depression | 12 | 7.2 (3.1−16.8) | <0.001 |
| Psychiatric disorder | 11 | 7.0 (3.1−15.5) | <0.001 |
| Neurogenic disorder | 9 | 4.9 (2.2−11.3) | <0.001 |
| QTc prolongation | 6 | 2.8 (1.3−5.7) | 0.006 |
Adapted from Ghadri et al. [25].
Detection of wall motion abnormalities by left ventriculography and echocardiography
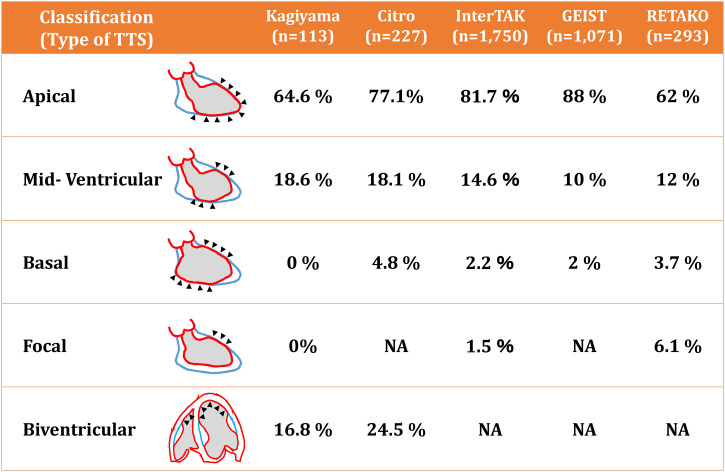
Although left ventriculography was originally used to detect its unique morphology mimicking “takotsubo”, echocardiography is currently an essential imaging modality to detect segmental as well as global wall motion abnormalities in patients with TTS. In addition to the apical ballooning (Fig. 2 ), there are several subtypes of TTS based on the location and extension of asynergy (Fig. 3 ). Prevalence of apical, mid-ventricular (Fig. 4 ), basal, and focal types are 62-88%, 10-18.5%, 0-5.8%, and 0-6.1%, respectively [[26], [27], [28], [29], [30], [31]]. Importantly, right ventricular wall motion could be involved with and without left ventricular wall motion abnormalities [6,26,27,32]. Because earlier studies used left ventriculography to detect and diagnose TTS, right ventricular involvement had not been reported until 2006, when two studies from the USA and EU reported right ventricular involvement in TTS [33,34]. In addition, a study using cardiac magnetic resonance imaging revealed the presence of right ventricular involvement in 81 of 239 (34%) TTS patients [35]. Presence of right ventricular involvement, or biventricular TTS, are associated with worse in-hospital as well as long-term clinical outcomes [26,27,36] (Fig. 5 ). Right ventricle may be involved without left ventricular wall motion abnormalities (isolated right ventricular TTS) [[37], [38], [39], [40], [41]].
Fig. 2.
Apical takotsubo syndrome. (A) Left ventriculography demonstrates typical apical ballooning, or “takotsubo”-like appearance. (B) Echocardiography demonstrates akinesis of the apical segment (arrows).
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Fig. 3.
Classification of takotsubo syndrome based on the location of wall motion abnormalities.
Adapted from Kagiyama et al. [26], Citro et al. [28], Templin et al. [29], Uribarri et al. [30], and Arcari et al. [31].
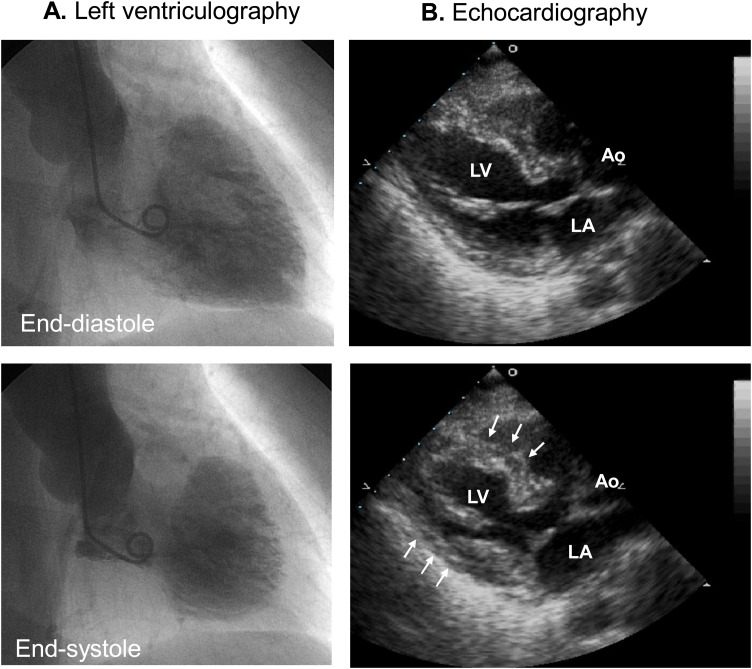
Fig. 4.
Mid-ventricular takotsubo syndrome. (A, B) Both left ventriculography and echocardiography demonstrated akinesis (arrows) of the mid-ventricular segment.
Ao, aorta; LA, left atrium; LV, left ventricle.
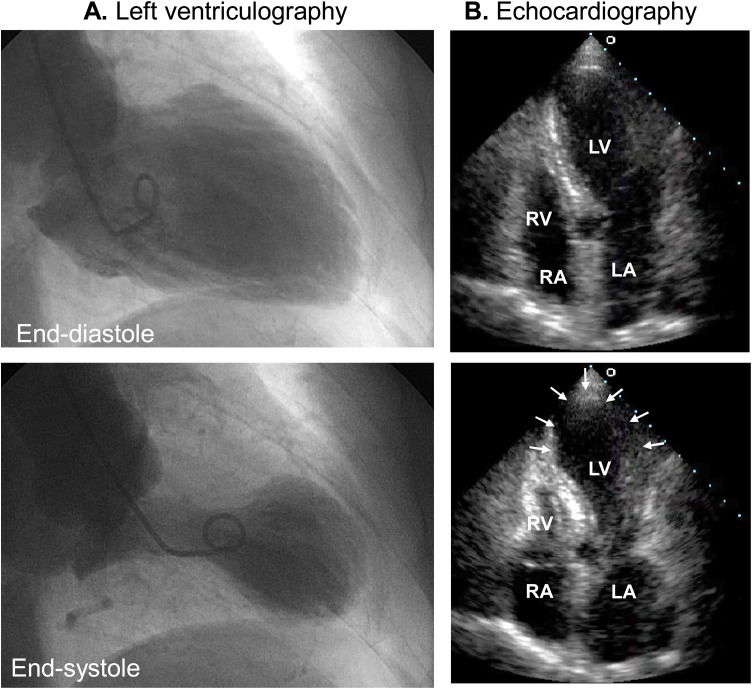
Fig. 5.
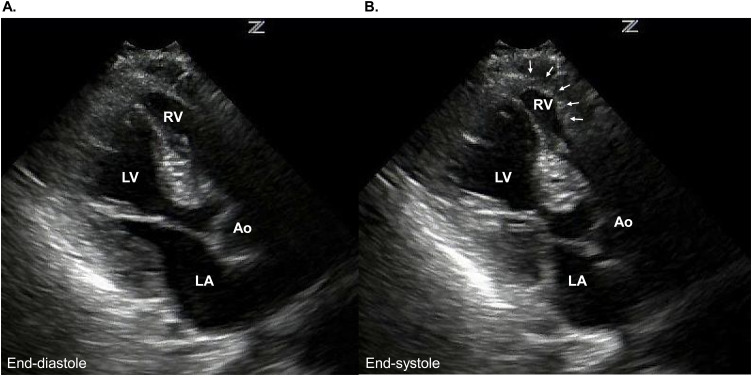
Biventricular takotsubo syndrome. Apical long-axis view of the echocardiography [end-diastole (A) and end-systole (B)] demonstrates akinesis of LV and RV (arrows).
Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
Unfavorable echocardiographic findings related to TTS
Echocardiography is useful to detect unfavorable findings related to TTS [6]. First, left ventricular outflow tract obstruction (LVOTO) may develop in TTS patients with apical ballooning and a hyperkinetic basal wall motion (Fig. 6 ). LVOTO is usually complicated in patients with hypertrophic obstructive cardiomyopathy or elderly patients with sigmoid septum [42].
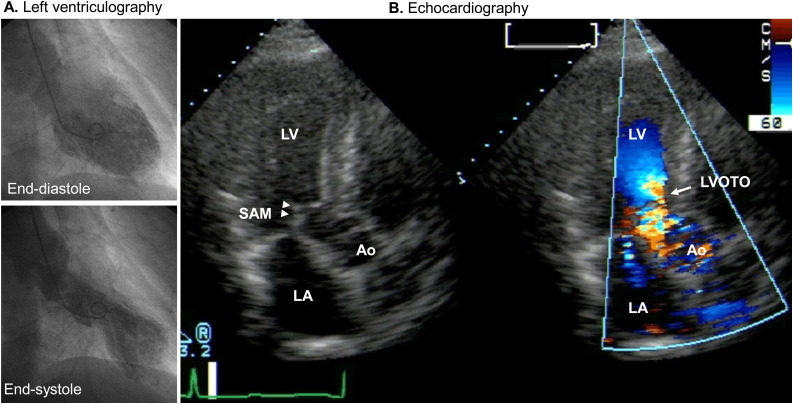
Fig. 6.
Left ventricular outflow tract obstruction (LVOTO) in takotsubo syndrome. (A) Left ventriculography shows apical ballooning. (B) Echocardiography shows LVOTO (arrow) and mitral regurgitation caused by systolic anterior motion (SAM) of the anterior mitral valve (arrow heads).
Ao, aorta; LA, left atrium; LV, left ventricle.
In TTS, preexisting septal bulge and hyperkinetic septal motion are possible causes of LVOTO. Prevalence of LVOTO among TTS patients ranges from 9.7% to 33% [27,[43], [44], [45], [46]]. LVOTO is an important cause of hypotension and heart failure during acute phase of TTS because use of positive inotropic agent and/or diuretics usually exaggerates LVOTO and as a result, deteriorate hemodynamic condition. Use of a beta-blocker, hydration in addition to cessation of positive inotropic agents, diuretics, or vasodilators are reasonable treatments for LVOTO. LVOTO sometimes causes acute mitral regurgitation [47] as a result of systolic anterior motion (SAM) of the anterior mitral leaflet, further deteriorating hemodynamic conditions. Prevalence of mitral regurgitation in TTS ranges from 15%-21.5% [27,28,48]. Acute mitral regurgitation is associated with major adverse events (a composite of acute heart failure, cardiogenic shock, and in-hospital mortality) [28] and therefore careful observation and treatment are mandatory. In addition to LVOTO, tethering of the mitral leaflet due to acute left ventricular dilatation may cause transient and significant mitral regurgitation [49]. Detection of the mechanisms of mitral regurgitation by echocardiography is important because of its implications for treatment. As mentioned above, diuretics, vasodilators, or positive inotropes should be avoided in patients with mitral regurgitation caused by LVOTO. On the other hand, mitral regurgitation caused by leaflet tethering may be better treated with such medications.
Left ventricular thrombus may develop during acute phase of TTS and its prevalence ranges from 2.2% to 8% [27,[50], [51], [52], [53]]. According to the largest study, 12 of 541 (2.2%) TTS patients had left ventricular thrombus and 2 strokes were documented before initiation of anticoagulation [53]. Oral anticoagulation is recommended until the left ventricular thrombus has resolved and the wall motion recovered.
A very rare but critical complication is left ventricular free wall or septal rupture [[54], [55], [56], [57], [58], [59], [60]]. Persistent ST elevation may be a high-risk electrocardiographic finding for left ventricular rupture [57].
Co-existence of coronary artery disease and TTS
In both Mayo criteria [21] and InterTak diagnostic criteria [22] significant CAD is not an exclusion criteria of TTS. Indeed, coexistence of acute coronary syndrome has been reported in 10-29% of TTS patients [29,61,62]. Acute coronary syndrome may not only coexist but also can trigger the TTS. In our recent study including 413 patients who were admitted to a cardiac care unit (CCU), we found 5 patients with acute myocardial infarction also had TTS based on retrospective review of the echocardiographic images [24]. Fig. 7 shows echocardiographic images from a patient with acute posterior myocardial infarction. Apical ballooning was clearly detected which cannot be explained by the left circumflex coronary artery lesion. As expected, presence of CAD in patients with TTS is a sign of worse prognosis. A recent study including 1016 TTS patients demonstrated that non-obstructive CAD was present in 23.0% and obstructive CAD in 41.2% of the TTS patients [63]. Presence of CAD was associated with increased incidence of shock, ventilation, and death from any cause. Furthermore, TTS patients with obstructive CAD were at comparable risk for shock and death and nearly at twice the risk for ventilation compared to an age- and sex-matched acute coronary syndrome cohort [63]. In addition to CAD, various cardiac diseases can trigger or coexist with TTS. In our above-mentioned study, aortic stenosis, cardiac amyloidosis, and tachycardia-induced cardiomyopathy coexisted or triggered TTS in CCU [24]. Fig. 8 is from a patient who was admitted to our hospital because of recurrent episodes of acute decompensated heart failure. An echocardiography at the previous admission demonstrated diffuse left ventricular hypertrophy and mildly depressed left ventricular systolic function and impaired left ventricular diastolic function. In echocardiography at the time of recurrent episode of heart failure (Fig. 8 A,B), we noticed biventricular apical asynergy that was not detected before. Coronary angiography was normal and technetium pyrophosphate scintigraphy demonstrated significant uptake suggestive of transthyretin cardiac amyloidosis (Fig. 8C). She was diagnosed with TTS complicated with transthyretin cardiac amyloidosis. A recent Japanese nationwide survey including 5274 patients with TTS revealed that 3255 (61.7%) underwent coronary angiography and 2019 (38.3%) did not [64]. Although prognosis between TTS with and without coronary angiography did not differ, TTS patients complicated with CAD may be underdiagnosed.
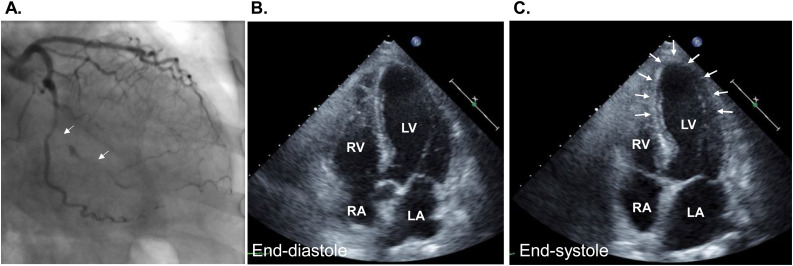
Fig. 7.
A case of takotsubo syndrome complicated with posterior myocardial infarction. (A) Coronary angiogram shows occlusion of the postero-lateral branch of the left circumflex coronary artery (arrows). (B, C) Echocardiography shows akinesis of the apical segment (arrows) that cannot be explained by the coronary artery occlusion.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
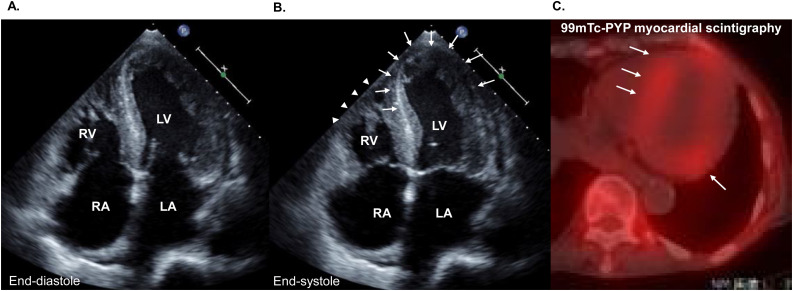
Fig. 8.
A case of takotsubo syndrome complicated with cardiac amyloidosis. (A, B) Echocardiography shows diffuse left ventricular hypertrophy and hypokinesis and akinesis of the left (arrows) and right ventricular (arrow heads) apical segments.
(B) 99 m Tc-pyrophosphate (99 m Tc-PYP) myocardial scintigraphy demonstrates significant uptake (arrows), suggesting the diagnosis of transthyretin cardiac amyloidosis.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Prognosis of TTS
As mentioned above, prognosis of TTS has been recognized as benign. However, recent studies consistently reported in-hospital mortality of 3.5-10.6% comparable to that of acute coronary syndrome [26,29,[65], [66], [67]]. Data from the Tokyo Coronary Care Unit Network including 107 patients with TTS demonstrated high in-hospital complications including 37 pump failure and 2 sustained ventricular tachycardia or fibrillation and 2 atrioventricular block [68]. As expected, TTS with cancer/malignancy had significantly lower survival [69,70]. Similarly, TTS triggered by physical stress had significantly lower survival than those by emotional stress or no stress [67,69]. InterTAK classification demonstrated that 5-year mortality is higher in class IIa [physical activities, medical conditions, or procedures, HR = 3.78 (95% CI: 2.21–6.44), p < 0.0001], class IIb [neurologic disorders, HR=5.76 (95% CI:2.96−11.2), p < 0.0001], and class III [no stress factor, HR=2.14 (95% CI:1.20−3.82), p = 0.010] as compared with class I (emotional stress) [67].
Although most cases of TTS are self-limiting, there are subsets of patients who develop recurrent episode of TTS [23,71]. Data from the Swedish Coronary Angiography and Angioplasty Register between 2009 and 2013 demonstrated that the mortality of TTS is worse than in control subjects without CAD and similar to patients with CAD [72]. Data from the InterTAK registry database demonstrated that a rate of death from any cause of 5.6% per patient-year and a rate of major adverse cardiac and cerebrovascular events of 9.9% per patient-year [29]. A recent meta-regression analysis including 4679 patients from 54 studies demonstrated that the annual rate of total mortality was 3.5% and that of recurrence was 1.0%, respectively [73].
Treatment of TTS
As of today, no randomized prospective study has been conducted to assess the prognostic impact of any specific medication on prognosis of TTS. Considering the possible role of catecholamine toxicity on TTS, it is reasonable to consider the use of beta-blockers. Isogai et al. compared 422 TTS patients who were treated with early beta-blocker therapy and 1688 propensity score matched controls and found that early beta-blocker use was not associated with lower 30-day mortality [74]. Results from the InterTAK registry demonstrated that survival was comparable between patients with and those without beta-blockers at discharge [29]. On the other hand, the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at discharge was associated with improved survival [29]. With respect of recurrence of TTS, a meta-analysis demonstrated that angiotensin-converting enzyme inhibitors or angiotensin receptor blockers rather than beta-blockers may reduce risk of recurrence [71]. A retrospective study suggested that antiplatelet therapy especially aspirin may be beneficial [75]. However, a recent larger scale study with longer (5 years) follow-up period demonstrated no association between aspirin use in TTS patients and a reduced risk of major adverse cardiovascular and cerebrovascular events at 30-day and 5-year follow-up [76].
COVID-19 and TTS
Currently, the pandemic of corona virus disease 19 (COVID-19) affects health status of the people all over the world. SARS-CoV-2 is a newly found corona virus that sometimes causes catastrophic respiratory failure requiring respirator and/or extracorporeal membrane oxygenation. It is reported that myocardial injury was present in 7.2% of patients with COVID-19 [77]. In particular, 22% of the COVID-19 patients admitted to intensive care unit had evidence of myocardial injury [77]. Importantly, myocardial injury is associated with worse prognosis of COVID-19 [78]. Acute myocarditis is one of the important causes of cardiac injury [[79], [80], [81], [82]]. In addition, several other cardiac complications may develop during the course of COVID-19 [82]. It is not surprising that TTS can develop in patients with COVID-19 considering its stressful physical as well as psychological situation. Meyer et al. first reported a typical case (apical ballooning) of TTS complicated with COVID-19 [83]. Both emotional stress and physical stress by infection itself are considered as possible triggers of typical [[83], [84], [85]] as well as atypical [86] TTS in patients with COVID-19 (Table 3 ). Not only the COVID-19 patient, but also his or her family may develop TTS possibly as a result of stressful situations [87]. During the diagnostic work up, care should be taken to keep sonographers and doctors safe [88,89]. Furthermore, it is recommended to perform coronary computed tomography rather than coronary angiography to rule out the presence of CAD [20].
Table 3.
Takotsubo syndrome associated with COVID-19.
| Age | Gender | Symptom | Electrocardiography | Type | |
|---|---|---|---|---|---|
| Case 1 | 83 | Female | Chest pain | ST elevation, T inversion | Apical |
| Case 2 | 67 | Female | Fever, cough | RBBB, T inversion | Apical |
| Case 3 | 52 | Male | Shortness of breath | ST elevation | Apical |
| Case 4 | 50 | Male | Chest pain | ST elevation | Mid-ventricular |
Disclosure
Nothing to disclose related to this article.
References
- 1.Iga K., Gen H., Tomonaga G., Matsumura T., Hori K. Reversible left ventricular wall motion impairment caused by pheochromocytoma--a case report. Jpn Circ J. 1989;53:813–818. doi: 10.1253/jcj.53.813. [DOI] [PubMed] [Google Scholar]
- 2.Iga K., Hori K. Rapidly progressive deteriorated left ventricular wall motion associated with tetanus: a case report. Jpn J Med. 1990;29:305–308. doi: 10.2169/internalmedicine1962.29.305. [DOI] [PubMed] [Google Scholar]
- 3.Dote K., Sato H., Tateishi H., Uchida T., Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases] J Cardiol. 1991;21:203–214. [PubMed] [Google Scholar]
- 4.Iga K., Hori K., Kitaguchi K., Matsumura T., Gen H., Tomonaga G. Transient segmental asynergy of the left ventricle of patients with various clinical manifestations possibly unrelated to the coronary artery disease. Jpn Circ J. 1991;55:1061–1067. doi: 10.1253/jcj.55.1061. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchihashi K., Ueshima K., Uchida T., Oh-mura N., Kimura K., Owa M. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 6.Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14:13–20. doi: 10.1007/s12574-015-0271-3. [DOI] [PubMed] [Google Scholar]
- 7.Ghadri J.R., Sarcon A., Diekmann J., Bataiosu D.R., Cammann V.L., Jurisic S. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur Heart J. 2016;37:2823–2829. doi: 10.1093/eurheartj/ehv757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kario K., Matsuo T. Increased incidence of cardiovascular attacks in the epicenter just after the Hanshin-Awaji earthquake. Thromb Haemost. 1995;74:1207. [PubMed] [Google Scholar]
- 9.Kario K., Ohashi T. Increased coronary heart disease mortality after the Hanshin-Awaji earthquake among the older community on Awaji Island. Tsuna Medical Association. J Am Geriatr Soc. 1997;45:610–613. doi: 10.1111/j.1532-5415.1997.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 10.Itabashi R., Furui E., Sato S., Yazawa Y., Kawata K., Mori E. Incidence of cardioembolic stroke including paradoxical brain embolism in patients with acute ischemic stroke before and after the Great East Japan Earthquake. Cerebrovasc Dis. 2014;37:431–437. doi: 10.1159/000363129. [DOI] [PubMed] [Google Scholar]
- 11.Sato K., Sakamoto K., Hashimoto Y., Hanzawa K., Sueta D., Kojima S. Risk factors and prevalence of deep vein thrombosis after the 2016 Kumamoto earthquakes. Circ J. 2019;83:1342–1348. doi: 10.1253/circj.CJ-18-1369. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe H., Kodama M., Okura Y., Aizawa Y., Tanabe N., Chinushi M. Impact of earthquakes on takotsubo cardiomyopathy. JAMA. 2005;294:305–307. doi: 10.1001/jama.294.3.305. [DOI] [PubMed] [Google Scholar]
- 13.Sato M., Fujita S., Saito A., Ikeda Y., Kitazawa H., Takahashi M. Increased incidence of transient left ventricular apical ballooning (so-called’ takotsubo’ cardiomyopathy) after the mid-Niigata Prefecture earthquake. Circ J. 2006;70:947–953. doi: 10.1253/circj.70.947. [DOI] [PubMed] [Google Scholar]
- 14.Wittstein I.S., Thiemann D.R., Lima J.A., Baughman K.L., Schulman S.P., Gerstenblith G. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 15.Kume T., Kawamoto T., Okura H., Toyota E., Neishi Y., Watanabe N. Local release of catecholamines from the hearts of patients with tako-tsubo-like left ventricular dysfunction. Circ J. 2008;72:106–108. doi: 10.1253/circj.72.106. [DOI] [PubMed] [Google Scholar]
- 16.Akashi Y.J., Nakazawa K., Sakakibara M., Miyake F., Musha H., Sasaka K. 123I-MIBG myocardial scintigraphy in patients with "takotsubo" cardiomyopathy. J Nucl Med. 2004;45:1121–1127. [PubMed] [Google Scholar]
- 17.Paur H., Wright P.T., Sikkel M.B., Tranter M.H., Mansfield C., O’Gara P. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of takotsubo cardiomyopathy. Circulation. 2012;126:697–706. doi: 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyon A.R., Rees P.S., Prasad S., Poole-Wilson P.A., Harding S.E. Stress (takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 19.Nakano T., Onoue K., Nakada Y., Nakagawa H., Kumazawa T., Ueda T. Alteration of beta-adrenoceptor signaling in left ventricle of acute phase takotsubo syndrome: a human study. Sci Rep. 2018;8:12731. doi: 10.1038/s41598-018-31034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Citro R., Okura H., Ghadri J.R., Izumi C., Meimoun P., Izumo M. Multimodality imaging in takotsubo syndrome: a joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE) J Echocardiogr. 2020 doi: 10.1007/s12574-020-00480-y. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Ghadri J.R., Wittstein I.S., Prasad A., Sharkey S., Dote K., Akashi Y.J. International Expert Consensus Document on Takotsubo Syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghadri J.R., Wittstein I.S., Prasad A., Sharkey S., Dote K., Akashi Y.J. International Expert Consensus Document on Takotsubo Syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okura H., Nakada Y., Ishihara S., Nogi M., Onoue K., Soeda T. "Hidden" takotsubo cardiomyopathy in cardiac care unit. J Echocardiogr. 2020;18:113–116. doi: 10.1007/s12574-019-00460-x. [DOI] [PubMed] [Google Scholar]
- 25.Ghadri J.R., Cammann V.L., Jurisic S., Seifert B., Napp L.C., Diekmann J. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail. 2017;19:1036–1042. doi: 10.1002/ejhf.683. [DOI] [PubMed] [Google Scholar]
- 26.Kagiyama N., Okura H., Tamada T., Imai K., Yamada R., Kume T. Impact of right ventricular involvement on the prognosis of takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:210–216. doi: 10.1093/ehjci/jev145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagiyama N., Okura H., Matsue Y., Tamada T., Imai K., Yamada R. Multiple unfavorable echocardiographic findings in takotsubo cardiomyopathy are associated with increased in-hospital events and mortality. J Am Soc Echocardiogr. 2016;29:1179–1187. doi: 10.1016/j.echo.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Citro R., Rigo F., D’Andrea A., Ciampi Q., Parodi G., Provenza G. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging. 2014;7:119–129. doi: 10.1016/j.jcmg.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Templin C., Ghadri J.R., Diekmann J., Napp L.C., Bataiosu D.R., Jaguszewski M. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 30.Uribarri A., Nunez-Gil I.J., Conty D.A., Vedia O., Almendro-Delia M., Duran Cambra A. Short- and long-term prognosis of patients with takotsubo syndrome based on different triggers: importance of the physical nature. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arcari L., Musumeci M.B., Stiermaier T., El-Battrawy I., Moller C., Guerra F. Incidence, determinants and prognostic relevance of dyspnea at admission in patients with takotsubo syndrome: results from the international multicenter GEIST registry. Sci Rep. 2020;10:13603. doi: 10.1038/s41598-020-70445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsugu T., Nagatomo Y., Nakajima Y., Kageyama T., Endo J., Itabashi Y. Biventricular takotsubo cardiomyopathy with asymmetrical wall motion abnormality between left and right ventricle: a report of new case and literature review. J Echocardiogr. 2019;17:123–128. doi: 10.1007/s12574-019-00424-1. [DOI] [PubMed] [Google Scholar]
- 33.Elesber A.A., Prasad A., Bybee K.A., Valeti U., Motiei A., Lerman A. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol. 2006;47:1082–1083. doi: 10.1016/j.jacc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Haghi D., Athanasiadis A., Papavassiliu T., Suselbeck T., Fluechter S., Mahrholdt H. Right ventricular involvement in takotsubo cardiomyopathy. Eur Heart J. 2006;27:2433–2439. doi: 10.1093/eurheartj/ehl274. [DOI] [PubMed] [Google Scholar]
- 35.Eitel I., von Knobelsdorff-Brenkenhoff F., Bernhardt P., Carbone I., Muellerleile K., Aldrovandi A. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 36.Finocchiaro G., Kobayashi Y., Magavern E., Zhou J.Q., Ashley E., Sinagra G. Prevalence and prognostic role of right ventricular involvement in stress-induced cardiomyopathy. J Card Fail. 2015;21:419–425. doi: 10.1016/j.cardfail.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Kagiyama N., Okura H., Kume T., Hayashida A., Yoshida K. Isolated right ventricular takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:285. doi: 10.1093/ehjci/jeu207. [DOI] [PubMed] [Google Scholar]
- 38.Sumida H., Morihisa K., Katahira K., Sugiyama S., Kishi T., Oshima S. Isolated right ventricular stress (takotsubo) cardiomyopathy. Intern Med. 2017;56:2159–2164. doi: 10.2169/internalmedicine.8323-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgdorf C., Hunold P., Radke P.W., Schunkert H., Kurowski V. Isolated right ventricular stress-induced ("tako-tsubo") cardiomyopathy. Clin Res Cardiol. 2011;100:617–619. doi: 10.1007/s00392-011-0293-4. [DOI] [PubMed] [Google Scholar]
- 40.Stahli B.E., Ruschitzka F., Enseleit F. Isolated right ventricular ballooning syndrome: a new variant of transient cardiomyopathy. Eur Heart J. 2011;32:1821. doi: 10.1093/eurheartj/ehr079. [DOI] [PubMed] [Google Scholar]
- 41.Elikowski W., Malek-Elikowska M., Rozanska P., Fertala N., Zawodna M. Isolated right ventricular takotsubo cardiomyopathy: a case report and literature review. Pol Merkur Lekarski. 2016;41:283–286. [PubMed] [Google Scholar]
- 42.Kobayashi S., Sakai Y., Taguchi I., Utsunomiya H., Shiota T. Causes of an increased pressure gradient through the left ventricular outflow tract: a West Coast experience. J Echocardiogr. 2018;16:34–41. doi: 10.1007/s12574-017-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozaki K., Okubo T., Tanaka K., Hosaka Y., Tsuchida K., Takahashi K. Manifestation of latent left ventricular outflow tract obstruction in the acute phase of takotsubo cardiomyopathy. Intern Med. 2016;55:3413–3420. doi: 10.2169/internalmedicine.55.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Backer O., Debonnaire P., Gevaert S., Missault L., Gheeraert P., Muyldermans L. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in takotsubo cardiomyopathy: a two-year, two-center experience. BMC Cardiovasc Disord. 2014;14:147. doi: 10.1186/1471-2261-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Mahmoud R., Mansencal N., Pilliere R., Leyer F., Abbou N., Michaud P. Prevalence and characteristics of left ventricular outflow tract obstruction in tako-tsubo syndrome. Am Heart J. 2008;156:543–548. doi: 10.1016/j.ahj.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Kawaji T., Shiomi H., Morimoto T., Tazaki J., Imai M., Saito N. Clinical impact of left ventricular outflow tract obstruction in takotsubo cardiomyopathy. Circ J. 2015;79:839–846. doi: 10.1253/circj.CJ-14-1148. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe N. Acute mitral regurgitation. Heart. 2019;105:671–677. doi: 10.1136/heartjnl-2018-313373. [DOI] [PubMed] [Google Scholar]
- 48.Haghi D., Rohm S., Suselbeck T., Borggrefe M., Papavassiliu T. Incidence and clinical significance of mitral regurgitation in takotsubo cardiomyopathy. Clin Res Cardiol. 2010;99:93–98. doi: 10.1007/s00392-009-0078-1. [DOI] [PubMed] [Google Scholar]
- 49.Izumo M., Shiota M., Nalawadi S., Das J., Dohad S., Kuwahara E. Determinants of secondary pulmonary hypertension in patients with takotsubo cardiomyopathy. Echocardiography. 2015;32:1608–1613. doi: 10.1111/echo.12949. [DOI] [PubMed] [Google Scholar]
- 50.de Gregorio C., Grimaldi P., Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with takotsubo-like syndrome: a systematic review. Int J Cardiol. 2008;131:18–24. doi: 10.1016/j.ijcard.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 51.Haghi D., Papavassiliu T., Heggemann F., Kaden J.J., Borggrefe M., Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako-tsubo cardiomyopathy assessed with echocardiography. QJM. 2008;101:381–386. doi: 10.1093/qjmed/hcn017. [DOI] [PubMed] [Google Scholar]
- 52.Kurisu S., Inoue I., Kawagoe T., Ishihara M., Shimatani Y., Nakama Y. Incidence and treatment of left ventricular apical thrombosis in tako-tsubo cardiomyopathy. Int J Cardiol. 2011;146:e58–60. doi: 10.1016/j.ijcard.2008.12.208. [DOI] [PubMed] [Google Scholar]
- 53.Santoro F., Stiermaier T., Tarantino N., De Gennaro L., Moeller C., Guastafierro F. Left ventricular thrombi in takotsubo syndrome: incidence, predictors, and management: results from the GEIST (German Italian Stress Cardiomyopathy) Registry. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S., Kaushik S., Nautiyal A., Choudhary S.K., Kayastha B.L., Mostow N. Cardiac rupture in takotsubo cardiomyopathy: a systematic review. Clin Cardiol. 2011;34:672–676. doi: 10.1002/clc.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shinozaki K., Tamura A., Abe Y., Yano S., Kadota J. Left ventricular free wall rupture in takotsubo cardiomyopathy. Int J Cardiol. 2007;115:e3–4. doi: 10.1016/j.ijcard.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 56.Kurisu S., Inoue I. Cardiac rupture in tako-tsubo cardiomyopathy with persistent ST-segment elevation. Int J Cardiol. 2012;158:e5–6. doi: 10.1016/j.ijcard.2011.10.059. [DOI] [PubMed] [Google Scholar]
- 57.Iskander M., Abugroun A., Shehata K., Iskander F., Iskander A. Takotsubo cardiomyopathy-induced cardiac free wall rupture: a case report and review of literature. Cardiol Res. 2018;9:244–249. doi: 10.14740/cr728w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akashi Y.J., Tejima T., Sakurada H., Matsuda H., Suzuki K., Kawasaki K. Left ventricular rupture associated with takotsubo cardiomyopathy. Mayo Clin Proc. 2004;79:821–824. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 59.Yamada R., Watanabe N., Kume T., Kawamoto T., Okahashi N., Wada N. Left ventricular rupture associated with takotsubo-like left ventricular dysfunction (apical ballooning) J Echocardiogr. 2006;4:59–62. [Google Scholar]
- 60.Tsuji M., Isogai T., Okabe Y., Nishimura Y., Itagaki S., Enatsu K. Ventricular septal perforation: a rare but life-threatening complication associated with takotsubo syndrome. Intern Med. 2018;57:1605–1609. doi: 10.2169/internalmedicine.0014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winchester D.E., Ragosta M., Taylor A.M. Concurrence of angiographic coronary artery disease in patients with apical ballooning syndrome (tako-tsubo cardiomyopathy) Catheter Cardiovasc Interv. 2008;72:612–616. doi: 10.1002/ccd.21738. [DOI] [PubMed] [Google Scholar]
- 62.Kurisu S., Inoue I., Kawagoe T., Ishihara M., Shimatani Y., Nakama Y. Prevalence of incidental coronary artery disease in tako-tsubo cardiomyopathy. Coron Artery Dis. 2009;20:214–218. doi: 10.1097/MCA.0b013e3283299260. [DOI] [PubMed] [Google Scholar]
- 63.Napp L.C., Cammann V.L., Jaguszewski M., Szawan K.A., Wischnewsky M., Gili S. Coexistence and outcome of coronary artery disease in takotsubo syndrome. Eur Heart J. 2020;41:3255–3268. doi: 10.1093/eurheartj/ehaa210. [DOI] [PubMed] [Google Scholar]
- 64.Isogai T., Matsui H., Tanaka H., Fushimi K., Yasunaga H. Clinical characteristics of patients with takotsubo syndrome diagnosed without coronary artery evaluation: a retrospective nationwide study. J Cardiol. 2018;71:268–276. doi: 10.1016/j.jjcc.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Brinjikji W., El-Sayed A.M., Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164:215–221. doi: 10.1016/j.ahj.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Almeida Jr G.L.G., Mansur Filho J., Albuquerque D.C., Xavier S.S., Pontes A., Gouvea E.P. Takotsubo Multicenter Registry (REMUTA) - Clinical aspects, in-hospital outcomes, and long-term mortality. Arq Bras Cardiol. 2020;115:207–216. doi: 10.36660/abc.20190166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghadri J.R., Kato K., Cammann V.L., Gili S., Jurisic S., Di Vece D. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Murakami T., Yoshikawa T., Maekawa Y., Ueda T., Isogai T., Konishi Y. Characterization of predictors of in-hospital cardiac complications of takotsubo cardiomyopathy: multi-center registry from Tokyo CCU Network. J Cardiol. 2014;63:269–273. doi: 10.1016/j.jjcc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Kim H., Senecal C., Lewis B., Prasad A., Rajiv G., Lerman L.O. Natural history and predictors of mortality of patients with takotsubo syndrome. Int J Cardiol. 2018;267:22–27. doi: 10.1016/j.ijcard.2018.04.139. [DOI] [PubMed] [Google Scholar]
- 70.Cammann V.L., Sarcon A., Ding K.J., Seifert B., Kato K., Di Vece D. Clinical features and outcomes of patients with malignancy and takotsubo syndrome: observations from the International Takotsubo Registry. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh K., Carson K., Usmani Z., Sawhney G., Shah R., Horowitz J. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int J Cardiol. 2014;174:696–701. doi: 10.1016/j.ijcard.2014.04.221. [DOI] [PubMed] [Google Scholar]
- 72.Tornvall P., Collste O., Ehrenborg E., Jarnbert-Petterson H. A case-control study of risk markers and mortality in takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67:1931–1936. doi: 10.1016/j.jacc.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 73.Pelliccia F., Pasceri V., Patti G., Tanzilli G., Speciale G., Gaudio C. Long-term prognosis and outcome predictors in takotsubo syndrome: a systematic review and meta-regression study. JACC Heart Fail. 2019;7:143–154. doi: 10.1016/j.jchf.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Isogai T., Matsui H., Tanaka H., Fushimi K., Yasunaga H. Early beta-blocker use and in-hospital mortality in patients with takotsubo cardiomyopathy. Heart. 2016;102:1029–1035. doi: 10.1136/heartjnl-2015-308712. [DOI] [PubMed] [Google Scholar]
- 75.Dias A., Franco E., Koshkelashvili N., Bhalla V., Pressman G.S., Hebert K. Antiplatelet therapy in takotsubo cardiomyopathy: does it improve cardiovascular outcomes during index event? Heart Vessels. 2016;31:1285–1290. doi: 10.1007/s00380-015-0729-2. [DOI] [PubMed] [Google Scholar]
- 76.D’Ascenzo F., Gili S., Bertaina M., Iannaccone M., Cammann V.L., Di Vece D. Impact of aspirin on takotsubo syndrome: a propensity score-based analysis of the InterTAK Registry. Eur J Heart Fail. 2020;22:330–337. doi: 10.1002/ejhf.1698. [DOI] [PubMed] [Google Scholar]
- 77.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paul J.F., Charles P., Richaud C., Caussin C., Diakov C. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging. 2020;21:776. doi: 10.1093/ehjci/jeaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou R. Does SARS-CoV-2 cause viral myocarditis in COVID-19 patients? Eur Heart J. 2020;41:2123. doi: 10.1093/eurheartj/ehaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inciardi Rm, Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer P., Degrauwe S., Van Delden C., Ghadri J.R., Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41:1860. doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sattar Y., Connerney M., Ullah W., Philippou A., Slack D., McCarthy B. COVID-19 presenting as takotsubo cardiomyopathy complicated with atrial fibrillation. Int J Cardiol Heart Vasc. 2020;29 doi: 10.1016/j.ijcha.2020.100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taza F., Zulty M., Kanwal A., Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Solano-Lopez J., Sanchez-Recalde A., Zamorano J.L. SARS-CoV-2, a novel virus with an unusual cardiac feature: inverted takotsubo syndrome. Eur Heart J. 2020;41:3106. doi: 10.1093/eurheartj/ehaa390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uhe T., Hagendorff A., Wachter R., Laufs U. Collateral damage: fear from SARS-CoV2-infection causing takotsubo cardiomyopathy. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01706-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brewer N., Huang G., Kwon Y. Sonographer safety issues during the COVID-19 pandemic. J Echocardiogr. 2020;18:197–198. doi: 10.1007/s12574-020-00474-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seo Y., Daimon M., Yamada H., Kagiyama N., Ohta M., Izumi C. Review of the efforts of the Japanese Society of Echocardiography for coronavirus disease 2019 (COVID-19) during the initial outbreak in Japan. J Echocardiogr. 2020 doi: 10.1007/s12574-020-00487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]