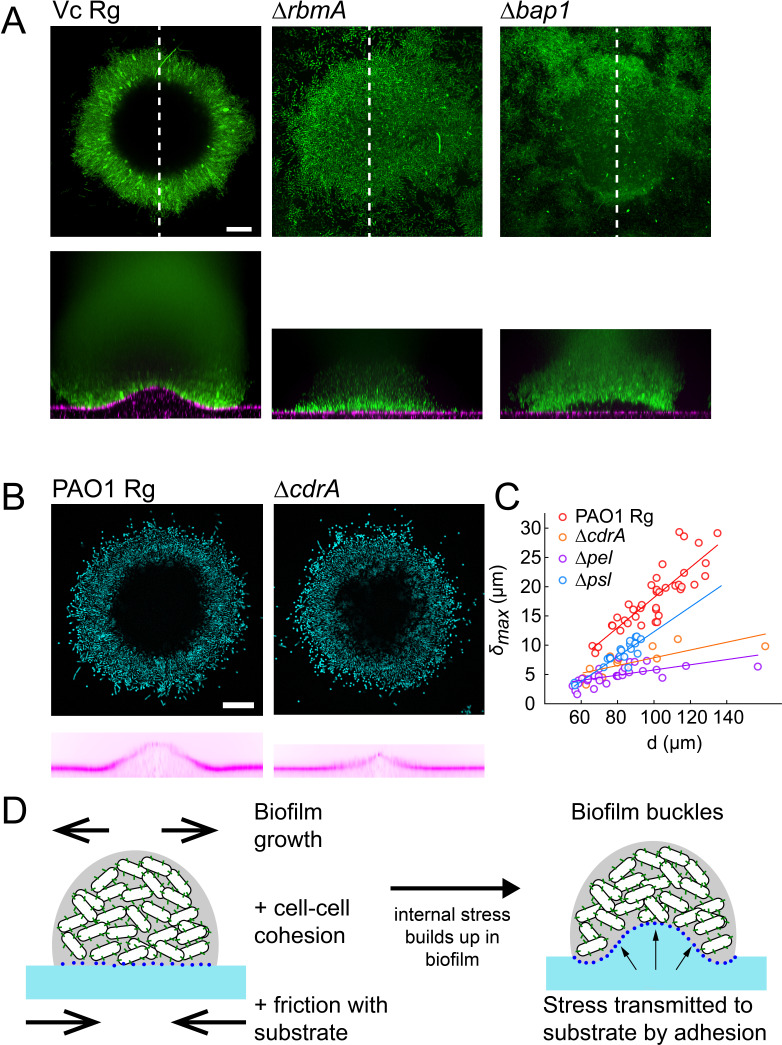
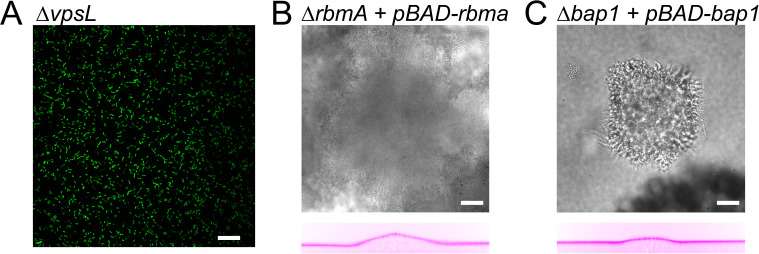
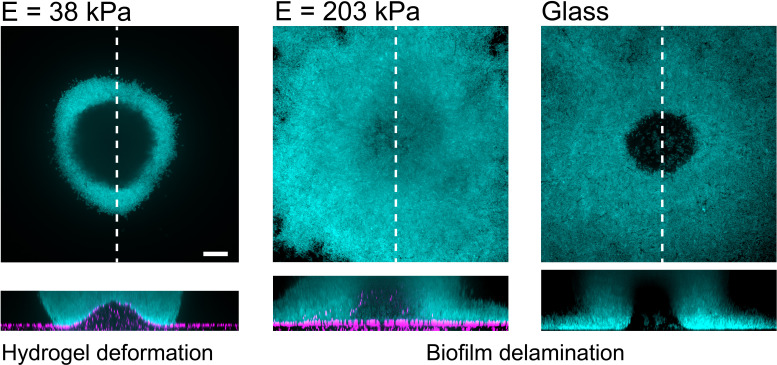
Figure 4. EPS composition drives biofilm and substrate deformations.
(A) Deformations of hydrogel substrates by V. cholerae Rg, rbma- and bap1- biofilms. Biofilms formed by rbma- and bap1- fail to deform the substrate. bap1- biofilms delaminate from the hydrogel surface. (B) Comparison of hydrogel deformations by P. aeruginosa Rg and cdrA- biofilms. (C) Dependence of maximum deformations on P. aeruginosa Rg, cdrA-, pel- and psl- biofilm diameter. All matrix mutants tend to generate weaker deformations compared to Rg. Data points correspond to different biofilms grown in two microfluidic chambers. (D) A model for the mechanism of biofilm deformation of soft substrates. Buildup of mechanical stress in the biofilm induces buckling. Adhesion between the biofilm and the surface transmits buckling-generated stress to the hydrogel, inducing deformations. E = 38 kPa. Scale bars: 20 µm.