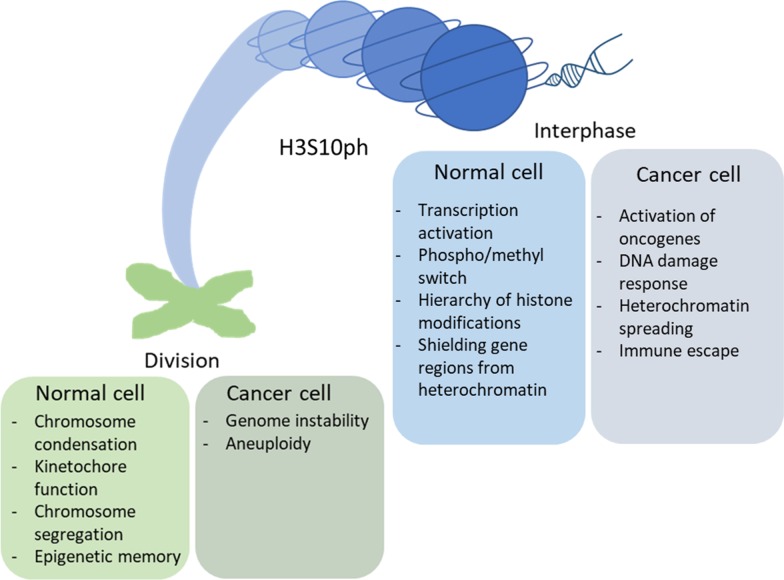
Fig. 2.
Effect of H3S10 phosphorylation on chromatin activity in normal and cancer cells. During interphase, enhancers-associated H3S10ph is involved in transcription activation and histone acetylation. By a ‘phospho-methyl switch’ it modulates epigenetic information coming from methylation of neighboring K9—by affecting H3K9me deposition it shields gene regions from heterochromatin. It also dictates spatiotemporal hierarchy of histone modifications. Deregulation of H3S10ph deposition can lead to cellular transformation via activation of protooncogenes, inhibition of developmental genes by heterochromatin spreading, genome instability caused by abnormal R-loops, and H3S10ph-mediated deregulation of inflammatory cytokines, potentially facilitating immune escape. During cell division H3S10ph is needed for proper chromosome condensation, kinetochore function and chromosome segregation. Deregulation of the deposition of this mark causes genome instability and aneuploidy caused by chromosome lagging or abnormal cytokinesis