Abstract
This is a rare case-report of a young female with systemic lupus erythematosus and end-stage kidney disease (on maintenance hemodialysis) who was admitted to our intensive care unit due to life-threatening COVID-19. The patient was diagnosed with a flare of lupus; while being on maintenance hydroxychloroquine therapy. However, after the administration of steroids she made an uneventful recovery and was discharged home. In this report, the diagnostic dilemmas and the therapeutic challenges due to the overlapping clinical, imaging, and laboratory findings between lupus and COVID-19 pneumonitis are outlined. In conclusion, patients with lupus may be affected by COVID-19 despite the administration of hydroxychloroquine. The administration of steroids may have a beneficial effect on mitigating both the flare of SLE and the COVID-19 associated hyperinflammation.
Keywords: COVID-19, Systemic lupus erythematosus, Acute respiratory failure, End-stage kidney disease, Steroid therapy, Hydroxychloroquine
1. Introduction
The novel coronavirus SARS-CoV-2 disease (COVID-19) emerged in Wuhan, the capital of Hubei province, in China, and progressively spread worldwide [[1], [2], [3]]. A minority of patients can develop fulminant disease, which is characterized by acute respiratory distress syndrome (ARDS), sepsis, thromboembolic disease, and multi-system organ failure [4,5]. Old age and underlying disorders such as arterial hypertension, diabetes mellitus, end-stage kidney disease (ESKD), and compromised immune status are risk factors affecting the morbidity and the mortality of COVID-19 patients [[6], [7], [8]]. The incidence of COVID-19 in patients with autoimmune disorders was not extensively studied. Also, great controversy exists about the potential role of hydroxychloroquine as protective therapy against COVID-19 in patients with systemic lupus erythematosus (SLE). Hydroxychloroquine was used in COVID-19 therapy due to its in vitro antiviral effects such as inhibition of the glycosylation of host receptors, and endosome acidification, amongst others, preventing likely the viral entry into the host cells (in vivo). However, the results in human trials were indeed controversial or even discouraging [[9], [10], [11]]. This report outlines diagnostic and treatment challenges in a young female COVID-19 patient with a flare of SLE.
2. Case presentation
A 28 year old female with a past medical history of SLE was admitted to the emergency department due to recent onset fever (38.8 °C), persistent cough, fatigue and progressive dyspnea. Also, she had ESKD due to lupus nephritis Grade VI, and being on maintenance hemodialysis via an arteriovenous fistula for the last three years. The patient's medications integrated hydroxychloroquine and mycophenolic acid as part of her SLE home management. Her mother revealed that she had protected (wearing a surgical mask) contact (one week ago) with one of her sisters who had just recovered from a flu-like illness. Since the endemic wave of COVID-19 had already affected Riyadh, the capital city of Saudi Arabia, we were alerted for a possible SARS-CoV-2 infection; hence, nasopharyngeal swabs were derived urgently and sent for testing. Moreover, we contacted the family physician requesting that her sister should be tested according to our Ministry of Health recommendations for COVID-19. Consequently, the sister was also tested positive for COVID-19 but was asymptomatic at that time. Hence, in our patient, the time period from symptoms onset to emergency department admission was approximately nine days.
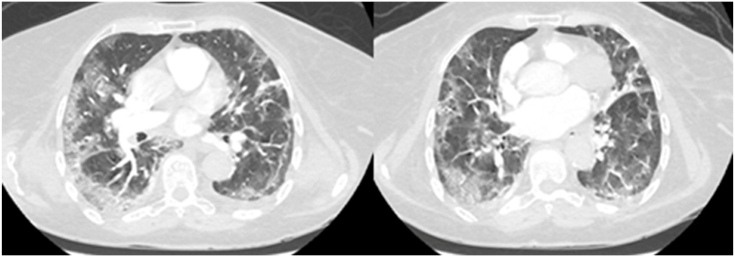
On physical examination decreased breath sounds and crepitations at the lung bases were evident. Her saturation of peripheral oxygen (SpO2) was 88%, on room air, and she had minor respiratory distress. She received 5 L of oxygen via a nasal cannula. Thereafter, the patient desaturated further (SpO2: 75%) and developed a picture of septic shock. Therefore, she was intubated and resuscitated by the intravenous administration of 500 ml of normal saline and low dose of noradrenaline to maintain a mean arterial pressure of 70 mm Hg. Electrocardiogram, cardiac enzymes, and echocardiography were normal. Laboratory findings were normal apart from lymphocytopenia (0.59 × 10⁹/l, normal: 1.1–3.2 × 10⁹/l), and increased C-reactive protein (354 mg/liter, normal: 0–7 mg/l), d-dimer (1.9 mcg/ml, normal: 0 to 0.5 mcg/ml), lactate dehydrogenase (737 units/l, normal: 100–190 units/l), and ferritin (1.126 ng/ml, normal: 23–336 ng/ml). Her blood urea nitrogen was 7.9 mmol/l (normal: 2.5–6.4 mmol/l) and creatinine 239 μmol/l (normal: 71–115 μmol/l). Emergency contrast chest computed tomography (CT) scan excluded pulmonary embolism but confirmed bilateral ground-glass opacities that were distributed in both the upper and lower lobes of the lung (Fig. 1). Subsequently, nasopharyngeal swabs confirmed COVID-19 by Real-Time-Polymerase-Chain-Reaction (RT-PCR) assays using QuantiNova Probe RT-PCR kit (Qiagen) in a Light-Cycler 480 real-time PCR system (Roche, Basel, Switzerland) [[12], [13], [14]].
Fig. 1.
Emergency contrast chest computed tomography scans revealing peripheral bilateral patchy ground-glass opacities with consolidations. Pulmonary embolism was excluded in our lupus patient with COVID-19.
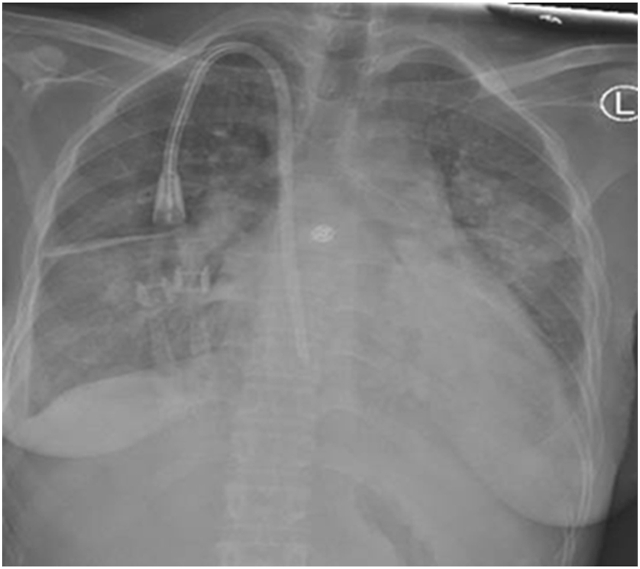
The patient was admitted to one of the 100 isolation chambers designated for COVID-19 supportive care within our 200-bed polyvalent intensive care unit (ICU). The patient underwent a full diagnostic work-up for other viral and bacterial infections. Also, the evaluation for a flare of SLE was initiated accordingly. The patient did not have any cutaneous manifestations of SLE or oral ulcers; furthermore, morning stiffness, and synovitis could not be properly assessed as the patient was intubated at that time. Laboratory tests showed elevated levels of anti-dsDNA at 22 IU/ml (normal values: <10 IU/mL) and decreased serum complement levels C3/C4 at 64 and 6 mg/dl, respectively [normal values: C3 (88–201 mg/dl) and C4 (10–40 mg/dl)]. No anti-cardiolipin antibodies, anti-β2GP1 antibodies, lupus anticoagulant or thrombocytopenia were detected. The diagnostic dilemma between rapidly evolving lupus pneumonitis and COVID-19 pneumonia emerged. Cultures were derived from all sites to investigate for any bacterial infections as the patient remained febrile. Bedside sonographic examination of the lower limbs excluded deep vein thrombosis. Empiric therapy for COVID-19 with lopinavir/ritonavir and ribavirin, piperacillin/tazobactam, as well as prophylactic anticoagulation along with sessions of hemodialysis every 48 hours, and supportive ICU care was administered as per hospital protocol [15]. Due to the fact that her fistula was malfunctioning a double lumen central line was inserted for hemodialysis (Fig. 2). Approximately three days post-ICU admission, the ratio of partial arterial pressure of oxygen to fractional inspired concentration of oxygen (SpO2/FiO2) was 150 despite the application of ARDS-net and prone positioning ventilation (positive end-expiratory pressure of 10 cm H20). On day-3, we decided the initiation of steroid treatment based on the pertinent clinical and laboratory data. After rheumatology consultation, we administered pulse methylprednisolone therapy (1 g/day intravenously) for three days (the patient's body weight was 60 kg). On day-4 post-ICU admission, the SpO2/FiO2 ratio increased to 300, and vasopressors were discontinued. On day-6, the patient was successfully extubated, and was placed on high flow nasal cannula (flow: 60 L/min, and FiO2 of 40%) maintaining SpO2 level over 96%. Notably, laboratory parameters improved as lymphocytes increased from 0.59 to 0.88 × 10⁹/l, and inflammatory biomarkers decreased: [C-reactive protein: from 354 to 37 mg/l; d-dimer: from 1.9 to 1 mcg/ml; lactate dehydrogenase: from 737 to 301 units/liter; and ferritin: from 1.126 to 381 ng/ml]. On day-8, the patient was placed on 2 L of oxygen via a nasal cannula without showing any respiratory distress. The results of all cultures for bacterial infections were negative. We continued her usual maintenance hemodialysis regime and steroid therapy. Oxygen supportive care was discontinued on day-10, and the methylprednisolone was tapered down to 60 mg per day (1mg/kg/day). RT-PCR test for COVID-19 and repeated microbiology tests were negative on day-17. The patient refused any follow-up testing, thus she was discharged to home isolation on day-19, on maintenance oral steroid therapy, and she is thereafter followed-up by her family physician.
Fig. 2.
Portable chest X-ray depicting bilateral interstitial infiltrates (the venous catheter is a double-lumen central line, which was inserted for hemodialysis as the patient's fistula was malfunctioning at that time).
3. Discussion
In this case-report, a puzzling clinical scenario is encountered. Our patient had COVID-19 pneumonia and a flare of SLE. Her presenting symptoms of fever, cough, dyspnea, hypoxia, and lung crepitations can be observed both in lupus and COVID-19 pneumonitis [[2], [3], [4], [5], [6], [7], [8],[16], [17], [18], [19]]. Moreover, lymhocytopenia can be a hallmark feature in both disorders [[4], [5], [6],20]. Apart from lymphocytopenia, other hematologic abnormalities including hemolytic anemia, leukopenia, and thrombocytopenia are included in the 2019 classification criteria for SLE by the American College of Rheumatology [20]. However, the aforementioned laboratory findings were not evident in our patient. Also, lupus pneumonitis could exhibit non-specific radiography and histology findings. Chest X-rays could demonstrate unilateral or bilateral infiltrates; while chest CT scans could depict ground-glass opacities. Findings on histology include alveolar wall damage, inflammatory cell infiltrates, edema, hemorrhage and necrosis [[16], [17], [18], [19]]. There is a significant overlap of findings between lupus and COVID-19 pneumonitis, and thus confusion may arise when trying to establish a diagnosis of lupus pneumonitis during the COVID-19 pandemic [[16], [17], [18], [19],21,22].
SLE patients with serious viral pneumonitis can be treated by the administration of steroids [23,24]. However, our COVID-19 patient with SLE had life-threatening features such as rapidly evolving septic shock and ARDS [[2], [3], [4], [5], [6], [7], [8]], which were not documented in previous reports [24]. ESKD might have at least partially contributed to the severity of the clinical picture, although not affecting unfavorably the outcome in our case [[2], [3], [4], [5], [6], [7], [8]]. Moreover, the standardized hemodialysis sessions along with the administration of other therapies, and the supportive ICU care may also contributed to the improvement of the patient's oxygenation. Antiviral agents such as remdesivir along with immunomodulatory therapies such as convalescent plasma transfusions, the new monoclonal antibody against interleukin-6 (tocilizumab), and plasma exchange showed promise in the treatment of COVID-19 but remain largely empiric [[25], [26], [27], [28], [29]].
The administration of steroids was not recommended in COVID-19, until recently, when the results of the beneficial role of low-dose dexamethasone therapy were reported in the RECOVERY trial [30]. In contrast, other recently published data showed no effect on 28-day mortality in critically ill COVID-19 patients after the administration of methylprednisolone (METCOVID trial), although patients over 60 years had a lower mortality in a subgroup analysis [31]. In our study, steroids were administered due to the flare of lupus in a young female patient. We are uncertain whether methylprednisolone mitigated as well the cytokine storm related to COVID-19 [[27], [28], [29],32,33]; however, given the severity of our patient's clinical picture it might have helped. The putative beneficial effect of steroids on viral pneumonitis was previously advocated in critically ill patients with Middle East Respiratory Syndrome [34]. This remains to be further studied in critically ill patients with COVID-19 along with the potential sequela of the administration of steroid therapy such as delaying of the viral clearance, and increasing the rate of secondary bacterial infections [[6], [7], [8]].
The most common signs and symptoms of lupus pneumonitis are fever, cough with lung crepitations and ensuing hypoxemia, which were also observed in our patient with SLE. Several pulmonary manifestations of SLE such as pleural and lung parenchymal involvement, pulmonary hypertension, diffuse alveolar hemorrhage, and thromboembolic disease, amongst others, are well documented [[16], [17], [18], [19], [20]]. However, lupus pneumonitis occurs only in a minority (1–4%) of SLE patients and carries a poor prognosis; while its therapy is still empiric. The basis of treatment is the administration of prednisone (1–1.5 mg/kg/day), and if there is no response, intravenous methylprednisolone (1g/day) can be added for three days [[16], [17], [18], [19], [20]]. The administration of steroid therapy, early in the course of lupus pneumonitis, is important due to its high mortality rate. If steroid therapy fails, other immunosuppressive or cytotoxic agents can be used. Fortunately, our patient responded favorably to the administration of steroid therapy.
Notably, this case-report underlines another trending controversy: the putative protective action of hydroxychloroquine in COVID-19. Recent data from the COVID-19 Global Rheumatology Alliance Registry recorded nineteen patients suffering from SLE out of the one hundred and ten rheumatologic patients who were diagnosed with COVID-19 [35]. Other studies provided evidence that the administration of hydroxychloroquine was not protective against COVID-19, which is in accordance with our current observations [36].
This case-report, albeit its many limitations that prevent its generalizability, carries important messages. Patients with SLE may be affected by COVID-19 despite the administration of hydroxychloroquine. The diagnostic dilemmas and the therapeutic challenges should be carefully processed due to the overlapping clinical, imaging, and laboratory findings between lupus and COVID-19 pneumonitis. In conclusion, the administration of steroids may have a beneficial effect on suppressing both the flare of SLE and the COVID-19 associated hyperinflammation. Further large prospective studies are required to confirm or refute the present findings.
CRediT authorship contribution statement
Abdulrahman Alharthy: Conceptualization, Investigation, Validation, Writing - original draft. Fahad Faqihi: Investigation, Validation, Writing - original draft. Nasir Nasim: Validation, Writing - original draft. Alfateh Noor: Investigation, Validation, Data Analysis. Saima Akhtar: Validation, Data Analysis. Ahmed Balshi: Investigation, Validation, Data Analysis, Writing - original draft. Abdullah Balhamar: Validation, Writing - original draft. Saleh A. Alqahtani: Supervision, review & editing, and revising the final draft. Ziad A. Memish: Validation, Supervision, review & editing the final draft. Dimitrios Karakitsos: Conceptualization, review & editing, and revising the final draft.
Declaration of competing interest
None.
Contributor Information
Abdulrahman Alharthy, Email: a_almshal@hotmail.com.
Fahad Faqihi, Email: dr.faqihi677@gmail.com.
Nasir Nasim, Email: drnasirnaseem1@hotmail.com.
Alfateh Noor, Email: alf.1000@yahoo.com.
Saima Akhtar, Email: drshehzadpk@yahoo.com.
Ahmed Balshi, Email: abalshi@hotmail.com.
Abdullah Balhamar, Email: abdullahbalahmar@gmail.com.
Saleh A. Alqahtani, Email: salqaht1@jhmi.edu.
Ziad A. Memish, Email: zmemish@yahoo.com.
Dimitrios Karakitsos, Email: karakitsosdimitrios@gmail.com.
Abbreviations
- SARS-CoV-2 disease
COVID-19
- ICU
intensive care unit
- RT-PCR
Real-Time-Polymerase-Chain-Reaction
- CT
computed tomography
- SLE
systemic lupus erythematosus
- ESKD
end-stage kidney disease
- ARDS
acute respiratory distress syndrome
- SpO2/FiO2 ratio
partial arterial pressure of oxygen to fractional inspired concentration of oxygen ratio
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study was approved by the Institutional Review Board of King Saud Medical City, Riyadh, Kingdom of Saudi Arabia [H-01-R-053, IORG0010374, H1RI-7-2020]. Written informed consent was obtained from the patient.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lu R., Zhao X., Li J. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 22 Feb 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. Epub 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Dingyu Z., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y., Wenzhe H., Yaowei H. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020:949–950. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 30 Apr 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 19 Feb 2019;368:m606. doi: 10.1136/bmj.m606. Feb 19: 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 6 Apr 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. 6 April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng Y., Liu W., Liu K. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: A retrospective study. Chin Med J (Engl) 5 Jun 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. J. Am. Med. Assoc. 12 May 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. Published online April 13. [DOI] [PubMed] [Google Scholar]
- 10.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 28 Jul 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. Published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W., Cao Z., Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomized controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalla A.K., Casto A.M., HuangMW Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J. Clin. Microbiol. 26 May 2020;58(6) doi: 10.1128/JCM.00557-20. April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WangW, Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 12 May 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. pii2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saudi Ministry of Health . 2020. Coronavirus Diseases 19 (COVID-19) Guidelines. (Revised Version 1.7) May 25th.https://covid19.moh.gov.sa [Google Scholar]
- 16.Hannah J.R., D'Cruz D.P. Pulmonary complications of systemic lupus erythematosus. Semin. Respir. Crit. Care Med. 2019;40:227–234. doi: 10.1055/s-0039-1685537. [DOI] [PubMed] [Google Scholar]
- 17.Keane M.P., Lynch J.P., 3rd Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax. 2000;55:159–166. doi: 10.1136/thorax.55.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan S.A., Teh C.L., Jobli A.T. Lupus pneumonitis as the initial presentation of systemic lupus erythematosus: case series from a single institution. Lupus. 2016;25:1485–1490. doi: 10.1177/0961203316646461. [DOI] [PubMed] [Google Scholar]
- 19.Aguilera-Pickens G., Abud-Mendoza C. Pulmonary manifestations in systemic lupus erythematosus: pleural involvement, acute pneumonitis, chronic interstitial lung disease and diffuse alveolar hemorrhage. Reumatol. Clínica. 2018;14:294–300. doi: 10.1016/j.reuma.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Aringer M., Costenbader K., Daikh D. 2019 European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2019;71:1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok C.C., Ying K.Y. Lupus pneumonitis or severe acute respiratory syndrome? Lupus. 2004;13:549–553. doi: 10.1191/0961203304lu1044cr. [DOI] [PubMed] [Google Scholar]
- 24.Kichloo A, Aljadah M, Albosta M et al. COVID-19 and acute lupus pneumonitis: diagnostic and treatment dilemma. Journal Invest Med High Imp Case Rep. Volume 8: 1–4. [DOI] [PMC free article] [PubMed]
- 25.Beigel J.H., Tomashek K.M., Dodd L.E. ACTT-1 study group members. Remdesivir for the treatment of covid-19 - preliminary report. N. Engl. J. Med. 2020 May 22 doi: 10.1056/NEJMoa2007764. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L., Zhang W., Hu Y., Tong X. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. J. Am. Med. Assoc. 2020 Jun 3 doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faqihi A., Alharthy A., Odat M. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: a pilot study. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.07.001. Jul 31:S0883-9441(20)30602-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 Jul;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horby P., Lim W.S., Emberson J.R. RECOVERY collaborative group, dexamethasone in hospitalized patients with covid-19 — preliminary report. N. Engl. J. Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [Google Scholar]
- 31.Jeronimo C.M.P., Farias M.E.L., Val F.F.A. For the metcovid team methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin. Infect. Dis. 2020 Aug 12:ciaa1177. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azkur A.K., Akdis M., Azkur D. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. Jul 2020;75(7):1564–1581. doi: 10.1111/all.14364. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 34.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 35.Robinson P.C., Yazdany J. The COVID-19 Global Rheumatology Alliance: collecting data in a pandemic. Nat. Rev. Rheumatol. Jun 2020;16(6):293–294. doi: 10.1038/s41584-020-0418-0. Published online April 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romão V.C., Cruz-Machado A.R., Fonseca J.E. No evidence so far on the protective effect of hydroxychloroquine to prevent COVID-19: response to the comment by Joob and Wiwanitkit. Ann. Rheum. Dis. 13 May 2020:217665. doi: 10.1136/annrheumdis-2020-217665. Published online May 13. [DOI] [PubMed] [Google Scholar]