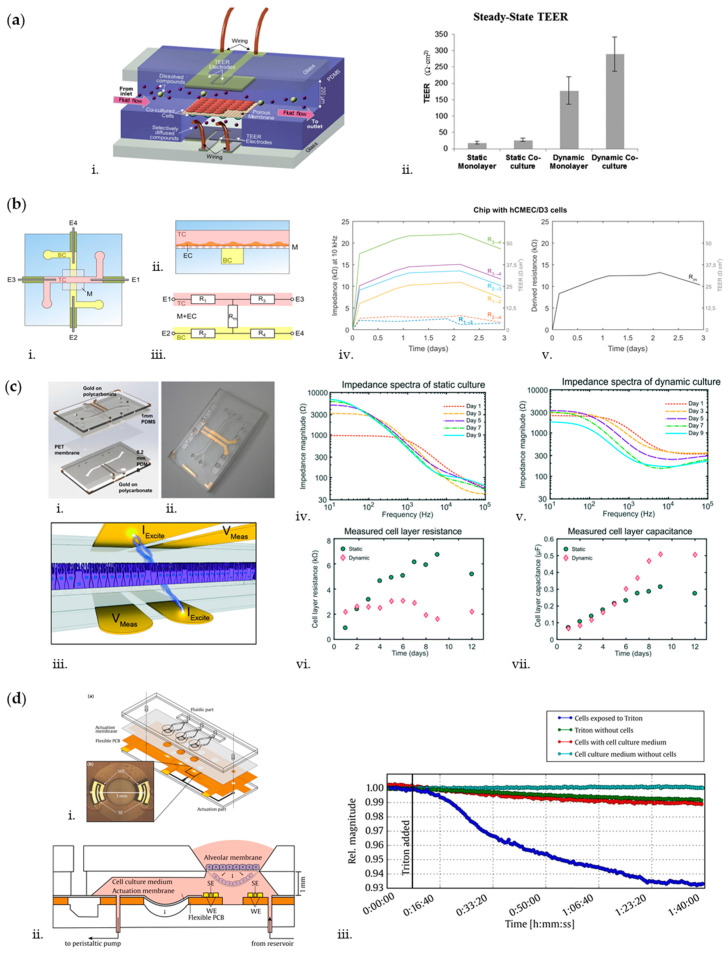
Figure 4.
(a) 3D illustration of the microfluidic blood–brain barrier (BBB) (i), composed of two perpendicular flow channels (lumenal on top and ablumenal at the bottom), separated at the channel junction by a porous membrane. Endothelial cells and astrocytes are, respectively, cultured on the lumenal and ablumenal sides of the membrane. Steady-state transepithelial/transendothelial electrical resistance (TEER) levels (ii) show that dynamic cultures (µBBB) reached significantly higher TEER levels than static cultures (Transwell controls). For both systems, cocultures of endothelial cells and astrocytes developed higher TEER levels than endothelial monolayers alone. Adapted from Ref. [108] with permission from The Royal Society of Chemistry. (b) Schematic top view (i) and side view (ii) of the chip to perform impedance spectroscopy measurements, showing top channel (TC), membrane (M), bottom channel (BC), platinum wire electrodes (E1, E2, E3, and E4), and endothelial cells (EC). The simplified equivalent circuit of the chip (iii) show resistors representing the top channel (R1 and R3), resistors representing the bottom channel (R2 and R4), and resistor Rm representing the membrane and EC barrier. Impedance and TEER measurements at 10 kHz for each electrode pair in a chip with hCMEC/D3 cells (iv) show a large variation between the electrode pairs. By calculating Rm (v) the resistance of the membrane and cells is isolated. Adapted from Ref. [115] with permission from Elsevier. (c) Exploded CAD (Computer-Aided Design) model (i) and photograph (ii) of the TEER-chip developed by Henry et al. Gold electrodes are patterned onto polycarbonate substrates. Laser cut polydimethylsiloxane (PDMS) layers and PET membrane are assembled using silane-based surface modification to irreversibly bond together. The schematic view of the chip and 4-point impedance measurement (iii). A small current of varying frequency is applied between two electrodes (Iexcite) located on each side of the cell monolayer, and the drop in potential between the second set of electrodes is measured (Vmeas). Impedance spectra obtained during the 12-days culture period of Caco-2 epithelial cells show the development of an intestinal epithelial barrier cultured under static (iv) or dynamic flow (v) conditions from days 1–9. The epithelial resistance (vi) of the statically cultured cell layer increased in time up to a plateau, while the resistance of the cell layer cultured under flow decreased after day 7. The capacitance (vii) of the static epithelium reached a plateau after day 8, while the capacitance of the epithelium cultured under dynamic flow conditions continued to increase. Adapted from Ref. [118] and Ref. [119] with permission from The Royal Society of Chemistry. (d) Design of the microimpedance tomography (MITO) and integration in the lung-on-chip (i). The MITO consists of a flexible printed circuit board (PCB) bonded between the actuation part and the actuation membrane of the lung-on-chip. It comprises three sensing regions, each consisting of a tetrapolar arrangement of two pairs of electrodes. The sensing electrodes (SE) of each pair are located in the center of the working electrodes (WE) to avoid zones of negative sensitivity. The cross-sectional view of the system (ii) shows a culture chamber containing the alveolar membrane, the actuation membrane, and the SE and WE of the MITO located 1 mm below the cell culture membrane. Microchannels connected to a reservoir enable the injection of solutions into the basal compartment. A time-lapse of the relative impedance magnitude at a frequency of 1 kHz (iii) reports changes in relative impedance of the bronchial epithelial monolayer after exposure to the permeabilization solution of 0.5% Triton X-100. Adapted from Ref. [121] with permission from Elsevier.