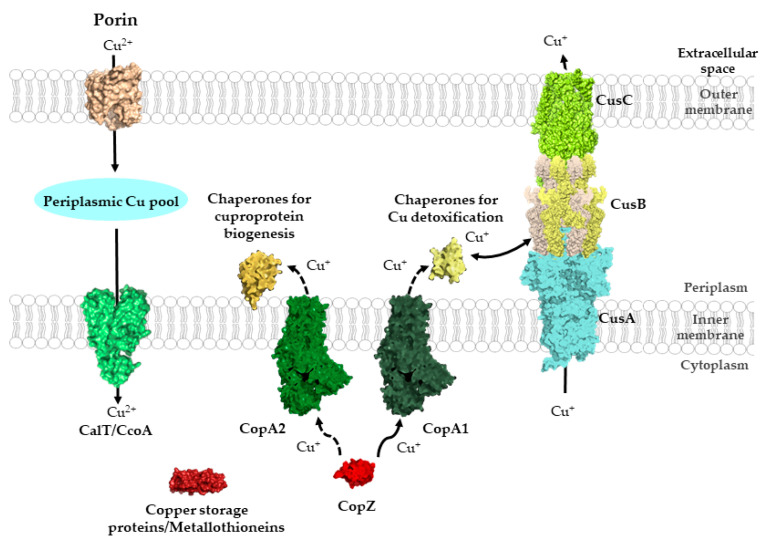
Figure 1.
General view of Cu transport across bacterial membranes. Cu can cross the outer membrane of bacteria via porins and major-facilitator superfamily members, such as CcoA, can import periplasmic Cu into the cytosol. Additional Cu importers likely exist but have not been characterized in detail. Cytosolic Cu is bound by Cu storage proteins, metallothioneines and Cu chaperones, such as CopZ. Cu-chaperones also deliver Cu to P1B-type ATPases for export into the periplasm. Kinetic differences distinguish CopA1-like ATPases, which are involved in Cu detoxification, and CopA2-like ATPases, which export Cu for cuproenzyme biogenesis. CopA1-like ATPases are the primary interaction partner of CopZ, while the interaction with CopA2-like ATPases is particularly important at low Cu concentrations (dashed arrow). In the periplasm, different types of chaperones transfer Cu either for cuproenzyme biogenesis or for Cu export systems, as with the CusABC system. The structures shown were retrieved from the protein database (PDB) with the following IDs: 2ZFG (OmpF for Porin), 3WDO (YajR for CalT/CcoA), 5NQO (Csp3 for copper storage proteins), 1K0V (CopZ), 3j09 (CopA1, CopA2) 4WBR (ScoI/SenC, chaperones for cuproprotein biogenesis), 2VB2 (CusF, chaperones for Cu detoxification), 3KSS (CusA), 3H94 (CusB), 4K7R (CusC), and are depicted using Pymol.