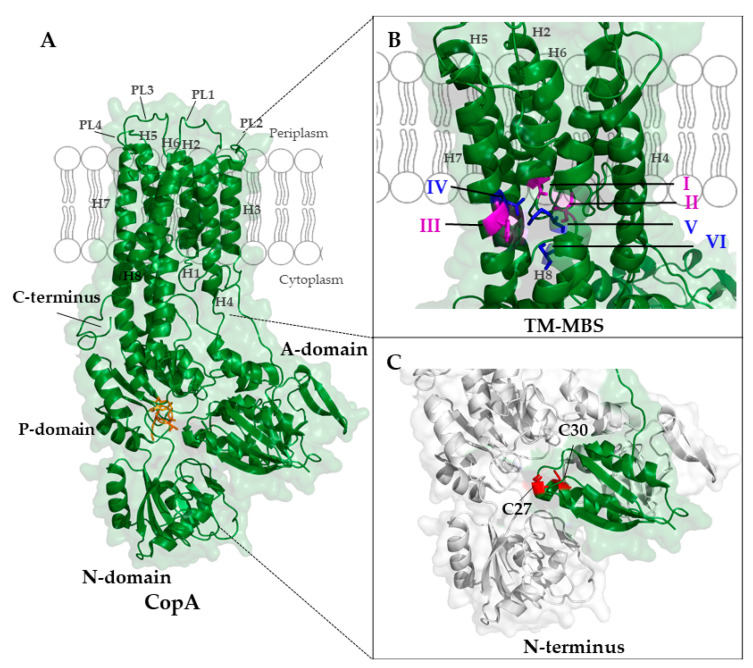
Figure 7.
Cryo-electron microscopy structure of the A. fulgidus CopA (PDB 3j09) [239]. (A) CopA contains eight transmembrane helices (H1-H8), four periplasmic loops (PL1-PL4) and three cytoplasmic domains (actuator domain (A-domain), nucleotide-binding domain (N-domain), and phosphorylation-domain (P-domain)). The invariant DKTGT motif of the P-domain is shown in orange. (B) The two transmembrane-metal-binding sites (TM-MBS) of CopA have both trigonal planar coordination geometries and are depicted in magenta (TM-MBS-1; Cys380 (I) and Cys382 (II) in helix 6 and Tyr682 (III) in helix 7)) and blue (TM-MBS-2; Asn 683 (IV) in helix 7, Met711 (V) and Ser715 (VI) in helix 8), respectively. (C) The N-terminus of CopA shows a ferredoxin-like βαββαβ-fold (green) with a typical CxxC motif (N-MBS-2); the two cysteines are highlighted in red. Note, that in the A. fulgidus CopA structure, the N-MBS-1 is not resolved. The structure of CopA was also determined by X-ray crystallography [57].