Table 2.
Forest BVOCs and their chemical characteristics listed on the basis of the magnitude of emissions (in descending order).
| Molecule | Chemical Family | IUPAC | Formula | Structure | CAS number | Boiling Point (at 760 mmHg) | Molar Mass (g/mol) | I/C 1 | C/D 2 | E 3 | P 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoprene | Isoprenoids | 2-methylbuta-1,3-diene | C5H8 |
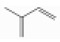
|
78-79-5 | 34.1 °C | 68.12 | C | D | **** | +++ |
| cis-3-Hexen-1-ol | GLVs | (Z)-hex-3-en-1-ol | C6H12O |
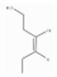
|
928-96-1 | 156.5 °C | 100.16 | I | D | *** | +++ |
| cis-3-Hexenal | GLVs | (Z)-hex-3-enal | C6H10O |
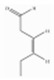
|
6789-80-6 | 126 °C | 98.14 | I | D | *** | +++ |
| cis-3-Hexenyl acetate | GLVs | [(Z)-hex-3-enyl] acetate | C8H14O2 |
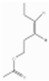
|
3681-71-8 | 174.2 °C | 142.2 | I | D | *** | +++ |
| d-Limonene | Monoterpene hydrocarbons | (4R)-1-methyl-4-prop-1-en-2-ylcyclohexene | C10H16 |
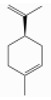
|
65996-98-7 | 175.4 °C | 136.23 | C, I | D | *** | +/+++ |
| α-Pinene | Monoterpene hydrocarbons | 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene | C10H16 |
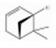
|
67762-73-6 | 156 °C | 136.23 | C, I | D | *** | +/+++ |
| (E)-β-Ocimene | Monoterpene hydrocarbons | (3E)-3,7-dimethylocta-1,3,6-triene | C10H16 |
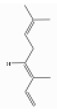
|
3779-61-1 | 175.2 °C | 136.23 | C, I | D | ** | +/++ |
| 1,8-Cineole | Monoterpenoid ethers | 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane | C10H18O |
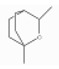
|
470-82-6 | 176.4 °C | 154.25 | C, I | D | ** | |
| Camphor | Monoterpenoid ketones | 1,7,7-trimethylbicyclo[2.2.1]heptan-2-one | C10H16O |
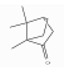
|
76-22-2 | 205.7 °C | 152.23 | ** | |||
| Linalool | Monoterpenoid alcohol | 3,7-dimethylocta-1,6-dien-3-ol | C10H18O |
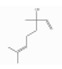
|
78-70-6 | 197.5 °C | 154.25 | C, L | D | ** | +/++ |
| p-Cymene | Aromatic monoterpene hydrocarbons | 1-methyl-4-propan-2-ylbenzene | C10H14 |
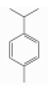
|
99-87-6 | 177 °C | 134.22 | C | D | ** | |
| Sabinene | Monoterpene hydrocarbons | 4-methylidene-1-propan-2-ylbicyclo[3.1.0]hexane | C10H16 |
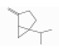
|
3387-41-5 | 164 °C | 136.23 | C | D | ** | |
| β-Caryophyllene | Sesquiterpene hydrocarbons | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | C15H24 |
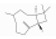
|
87-44-5 | NA | 204.35 | C, I | D | ** | +/++ |
| β-Myrcene | Monoterpene hydrocarbons | 7-methyl-3-methylideneocta-1,6-diene | C10H16 |
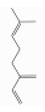
|
123-35-3 | 167 °C | 136.23 | C | D | ** | |
| β-Pinene | Monoterpene hydrocarbons | 6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptane | C10H16 |
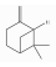
|
127-91-3 | 166.0 °C | 136.234 | C, I | D | ** | |
| β 3-Carene | Monoterpene hydrocarbons | 3,7,7-trimethylbicyclo[4.1.0]hept-3-ene | C10H16 |
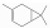
|
74806-04-5 | 171.4 °C | 136.234 | C | D | ** | |
| (E)-Linalool-oxide | Monoterpenoid oxide | 2-[(2S,5S)-5-ethenyl-5-methyloxolan-2-yl]propan-2-ol | C10H18O2 |
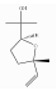
|
11063-78-8 | NA | 170.25 | C | D | * | |
| (Z)-Linalool-oxide | Monoterpenoid oxide | 2-(5-ethenyl-5-methyloxolan-2-yl)propan-2-ol | C10H18O2 |
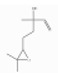
|
14049-11-7 | 224.2 °C | 170.25 | C | D | * | |
| Borneol | Monoterpenoid alcohol | 1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol | C10H18O |
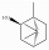
|
464-45-9 | 212.0 °C | 154.25 | C | * | ||
| Bornyl acetate | Monoteropene-derived ester | (1,7,7-trimethyl-2-bicyclo[2.2.1]heptanyl) acetate | C12H20O2 |
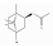
|
20347-65-3 | 223.5 °C | 196.286 | C | * | ||
| Camphene | Monoterpene hydrocarbons | 2,2-dimethyl-3-methylidenebicyclo[2.2.1]heptane | C10H16 |
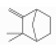
|
79-92-5 | 157.5 °C | 136.234 | C | D | * | |
| Terpinen-4-ol | Monoterpenoid alcohol | 4-methyl-1-propan-2-ylcyclohex-3-en-1-ol | C10H18O |
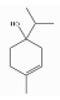
|
562-74-3 | 209.0 °C | 154.25 | C | * | ||
| α-Copaene | Sesquiterpene hydrocarbons | (1R)-1,3-dimethyl-8-propan-2-yltricyclo[4.4.0.02,7]dec-3-ene | C15H24 |
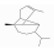
|
3856-25-5 | 248.5 °C | 204.35 | I | * | ||
| α-Humulene | Sesquiterpene hydrocarbons | (1E,4E,8E)-2,6,6,9-tetramethylcycloundeca-1,4,8-triene | C15H24 |
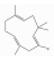
|
6753-98-6 | 166-168 °C | 204.35 | C | D | * | |
| α-Phellandrene | Monoterpene hydrocarbons | 2-methyl-5-propan-2-ylcyclohexa-1,3-diene | C10H16 |
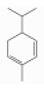
|
99-83-2 | 171.5 °C | 136.234 | C | * | ||
| α-Terpinene | Monoterpene hydrocarbons | 1-methyl-4-propan-2-ylcyclohexa-1,3-diene | C10H16 |
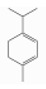
|
99-86-5 | 174.1 °C | 136.234 | C | D | * | |
| α-Terpineol | Monoterpenoid alcohol | 2-(4-methylcyclohex-3-en-1-yl)propan-2-ol | C10H18O |
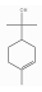
|
10482-56-1 | 217.5 °C | 154.249 | C | * | ||
| α-Terpinolene | Monoterpene hydrocarbons | 1-methyl-4-propan-2-ylidenecyclohexene | C10H16 |
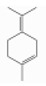
|
1124-27-2 | 186.0 °C | 138.25 | C | D | * | |
| β-Phellandrene | Monoterpene hydrocarbons | 3-methylidene-6-propan-2-ylcyclohexene | C10H16 |
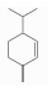
|
555-10-2 | 175 °C | 136.234 | C, I | * | +/+++ | |
| β-Terpinene | Monoterpene hydrocarbons | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | C10H16 |
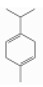
|
99-85-4 | 183.0 °C | 136.234 | C, I | D | * | |
| (Z)-β-Ocimene | Monoterpene hydrocarbons | (3Z)-3,7-dimethylocta-1,3,6-triene | C10H16 |
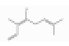
|
13877-91-3 | 175.2 °C | 136.234 | C, I | D | +/++ | |
| Bergamotene | Sesquiterpene hydrocarbons | 6-methyl-2-methylidene-6-(4-methylpent-3-enyl)bicyclo[3.1.1]heptane | C15H24 |
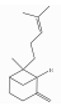
|
7663-66-3 | NA | 208.38 | I | |||
| DMNT | Homoterpene hydrocarbons | 4,8-dimethylnona-1,3,7-triene | C11H18 |
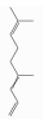
|
19945-61-0 | 195.6 °C | 150.26 | I | +/++ | ||
| Longifolene | Sesquiterpene hydrocarbons | 3,3,7-trimethyl-8-methylidenetricyclo[5.4.0.02,9]undecane | C15H24 |
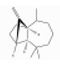
|
475-20-7 | 252.2 °C | 204.35 | C | D | ||
| Methyl jasmonate | Jasmonate ester | methyl 2-[(1R,2R)-3-oxo-2-[(Z)-pent-2-enyl]cyclopentyl]acetate | C13H20O3 |
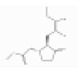
|
39924-52-2 | 302.9 °C | 224.296 | I | |||
| Methyl salicylate | Benzoate ester | methyl 2-hydroxybenzoate | C8H8O3 |
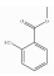
|
119-36-8 | 222.0 °C | 152.147 | I | ++++ | ||
| TMTT | Homoterpene hydrocarbons | (3E,7Z)-4,8,12-trimethyltrideca-1,3,7,11-tetraene | C16H26 |

|
62235-06-7 | 293.2 °C | 218.38 | I | +/++ | ||
| α-Thujene | Monoterpene hydrocarbons | 2-methyl-5-propan-2-ylbicyclo[3.1.0]hex-2-en | C10H16 |
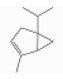
|
2867-05-2 | 152 °C | 136.23 | C | |||
| β-Farnesene | Sesquiterpene hydrocarbons | 7,11-dimethyl-3-methylidenedodeca-1,6,10-triene | C15H24 |
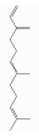
|
18794-84-8 | 279.6 °C | 204.35 | I | ++ |
1 I/C = Inducible/constitutive: forest VOC types. 2 C/D = Conifers/deciduous: tree types. 3 E = Emissions [3,8,42]: highly abundant emissions: ****; abundant emissions: ***; moderately abundant emissions: **; common emissions: *. 4 P = Persistence [3,8,42]: less than 10 min: +; 10–60 min: ++; 1 h–24 h: +++; more than 24 h: ++++.
