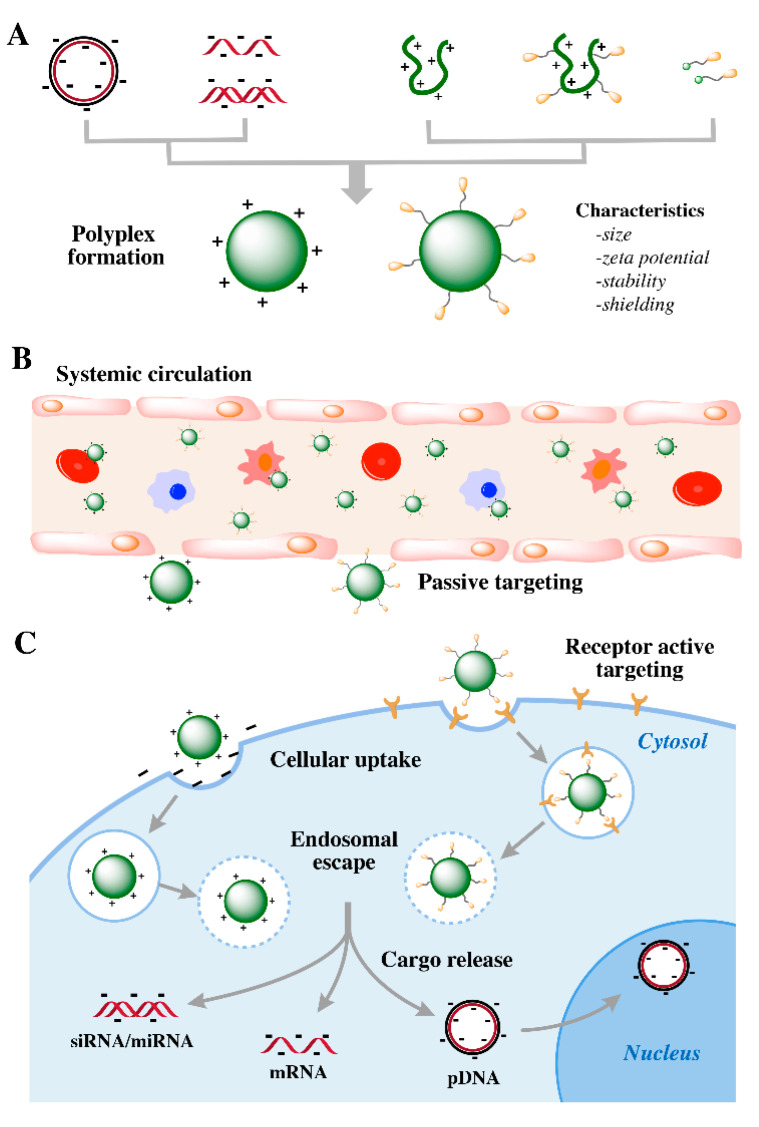
Figure 4.
Pharmacological barriers for systemically administered non-viral targeted nucleic acid delivery. (A) Production of stable nucleic acids-loaded polyplexes. (B) After intravenous injection, nanocarriers shall avoid unspecific interactions with blood components and rapid clearance, accumulating in targeting areas during circulation. (C) After the passage of fenestrated blood vessels, nanocarriers shall be internalized by target cells via an active transport process, and realize efficient endosomal escape and release the cargos in an active form for gene expression or regulation.