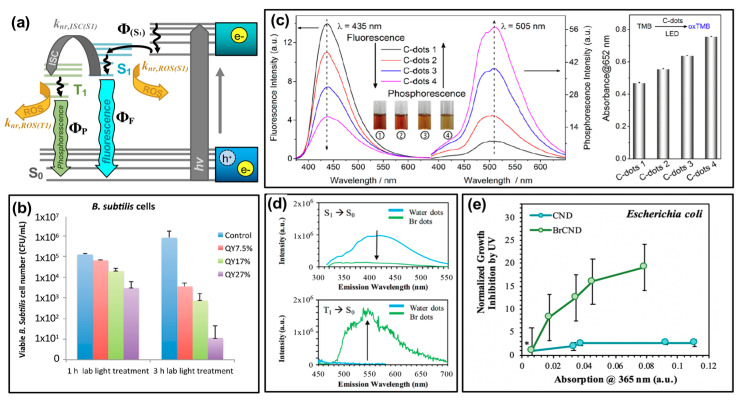
Figure 6.
Tuning the quantum yield (Φ) values of carbon nanodots for optimized antimicrobial activity. (a) Modified Jablonski diagram showing initial absorption by carbon nanodots and subsequent transition to an emissive excited state (ΦS1), whereby the state may radiatively relax (ΦF) or undergo non-radiative transitions including intersystem crossing (“ISC”, knr,ISC(S1)) to an excited triplet state (T1) or generation of reactive oxygen species (ROS). (b) Viability of Bacillus subtilis after treatment with light-activated carbon nanodots of varying fluorescent quantum yield, “QY”. (c) Left—Fluorescence and phosphorescence emission spectra for comparable carbon nanodot structures displaying variable quantum yield values (C-dots 1–4). Right—Detection of reactive oxygen species by TMB (3,3′,5,5′-tetramethylbenzidine) absorption increases, correlating to the phosphorescence quantum yield of varying carbon dot species. (d) Top—Fluorescence decrease from carbon dot samples (CND/“water dots”) following bromination (BrCND/“Br dots”) and bottom—corresponding changes in phosphorescence for the same samples. (e) Growth inhibition of Escherichia coli for phosphorescent carbon dots (BrCND) versus fluorescent carbon dots (CND) of varying concentrations (reported by corresponding absorption values); values were normalized to those for a non-BrCND-containing solution (*), from Reference [56]. For additional details regarding the reported studies, the reader is referred to Table 1 of the main text. Abbreviations: knr,ISC(S1) (rate of ISC from S1 excited state), knr,ROS(T1) (rate of ROS generation from triplet states), knr,ROS(S1) (rate of ROS generation from singlet states), oxTMB (oxidized TMB). Permissions: (b) Adapted from Reference [24]—Published by The Royal Society of Chemistry. (c) Adapted with permission from Reference [55]. Copyright © 2018, American Chemical Society. (d) Adapted from Reference [46]—Published by the PCCP Owner Societies.