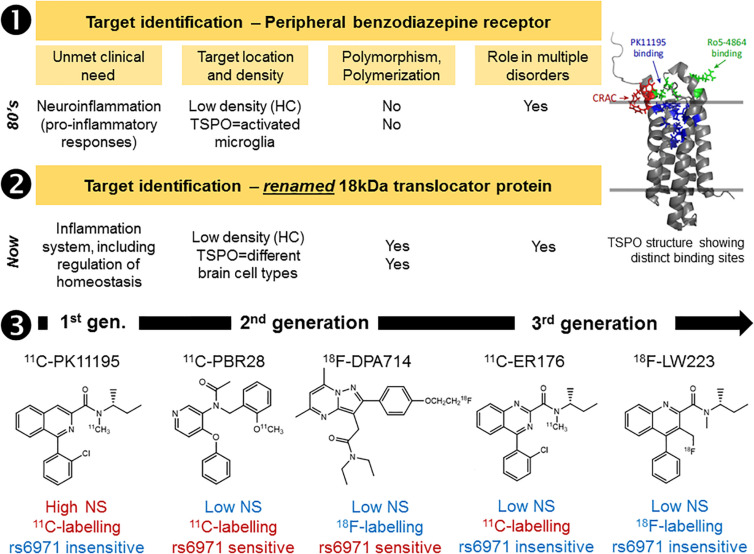
FIGURE 2.
Case study 1: Imaging the 18kDa translocator protein (TSPO), a “moving target” over the past three decades. The development of novel TSPO radiotracers suffered from high degree of attrition over the years (1–3). First there was the renaming of the target, in recognition of the wider functions of this protein. Then the identification of a genetic polymorphism (rs6971) capable of impacting on radiotracer binding in humans (2nd generation, 2nd gen., radiotracers). Alongside these discoveries came the recognition that TSPO has at least three binding sites and can be expressed in a monomer or a polymer configuration depending on the organ or disease process under investigation. Furthermore, limited characterization of the TSPO expression in various cell types decades ago has created a delayed realization that TSPO PET/SPECT imaging was always directed toward understanding TSPO molecular changes rather than cell changes. All these serendipitous findings at the first stage of the radiotracer development pipeline (target identification) have impacted on the development of novel and successful TSPO radiotracers (1 and 2). Despite these difficulties, through a series of compound libraries from 1st generation (1st gen.) radiotracers, like 11C-PK11195, to 3rd generation (3rd gen.) radiotracers like 11C-ER176 and 18F-LW223, it was possible to resolve the target identification issue, while improving radiotracer properties, such as reduction of non-specific binding (NS) and convenient labeling with fluorine-18 (3). Red and blue text indicates limitations and advantages of each example radiotracer displayed in row 3. HC, healthy control brain. TSPO structure taken from Selvaraj and Stocco, Trends in Endocrinology and Metabolism, 2015, 26(7):341.