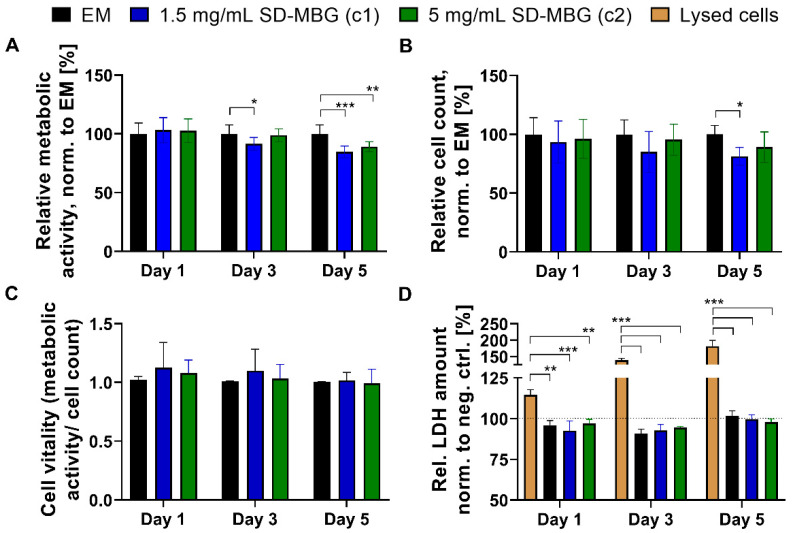
Figure 3.
Cytocompatibility upon indirect exposure of hMSCs to SD-MBGs for one, three and five days. (A) Metabolic activity using Presto Blue and measuring substrate conversion by the cells. (B) Cell count as determined by DAPI-staining, imaging of the wells (two 3 × 3 mosaic images per well using 10× magnification taken at slightly ex-centrical positions) and counting the nuclei using FIJI ImageJ software. (C) Cell vitality calculated by building a ratio of metabolic activity and cell count, indicating the viability per cell. (D) Relative lactate dehydrogenase (LDH) content in the supernatant compared to fresh culture medium serving as negative control and set to 100%. Cells cultured in expansion medium (EM) were lysed at each testing time point and were used as positive control to estimate the maximal amount of LDH that could be secreted into the supernatant. Normalization per time point as stated in the axis titles, n = three hMSC from different donors with four technical replicates each. One-way ANOVA with Dunnett’s multiple comparison test was performed using the positive control (EM: (A–C); lysed cells: (D)) as comparator. EM: cells cultured in expansion medium, hMSCs: human mesenchymal stromal cells, SD-MBG: spray-dried mesoporous bioactive glasses, c1: concentration 1 (1.5 mg/mL), c2: concentration 2 (5 mg/mL), DAPI: 4′,6-diamidino-2-phenylindole. p-values for statistical significance were * p < 0.05, ** p < 0.01, *** p < 0.001.