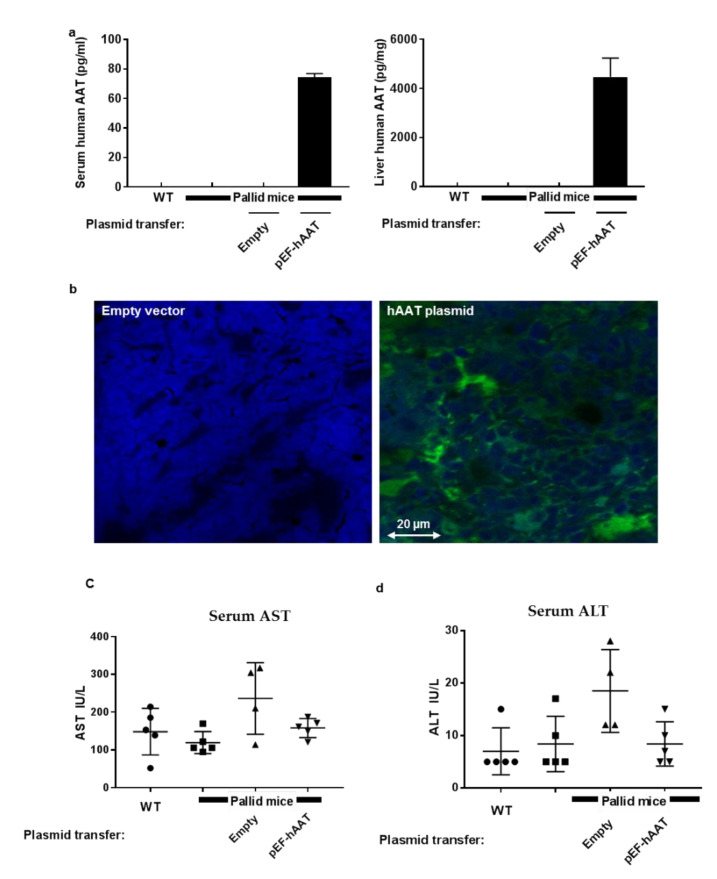
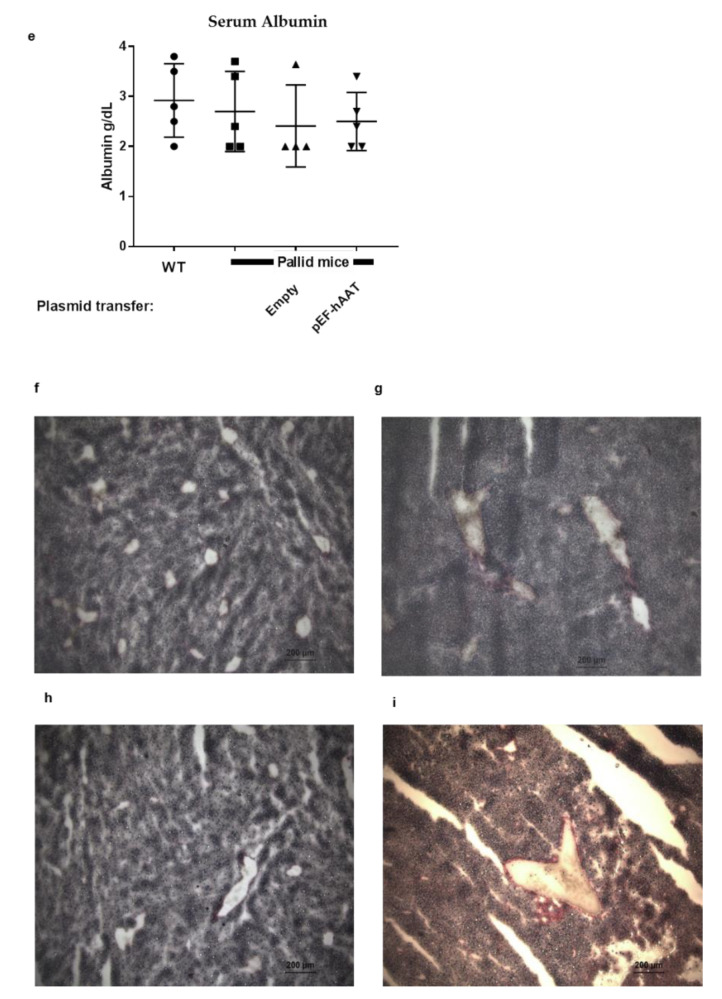
Figure 1.
Human alpha1 antitrypsin (AAT) expression 30 days after in vivo intrahepatic electroporation-mediated hAAT gene transfer: (a) hAAT levels in serum and liver homogenates, mean ± SD; (b) representative immunofluorescent images of the liver 30 days after intrahepatic electroporation-mediated gene transfer, green fluorescence: hAAT. Electroporation-mediated gene transfer is safe in the liver. Serum levels of liver enzymes were measured: (c) serum aspartate aminotransferase (AST), (d) serum alanine aminotransferase (ALT), and (e) serum albumin. Values are expressed as mean and ± SD; all the values are within physiological range. Sirius red staining of the liver section did not show any sign of liver injury or fibrosis after electroporation-mediated gene transfer as observed by Ishak system staining [23]. Wildtype (f), pallid (g), empty vector (h), and pEF-hAAT (i).