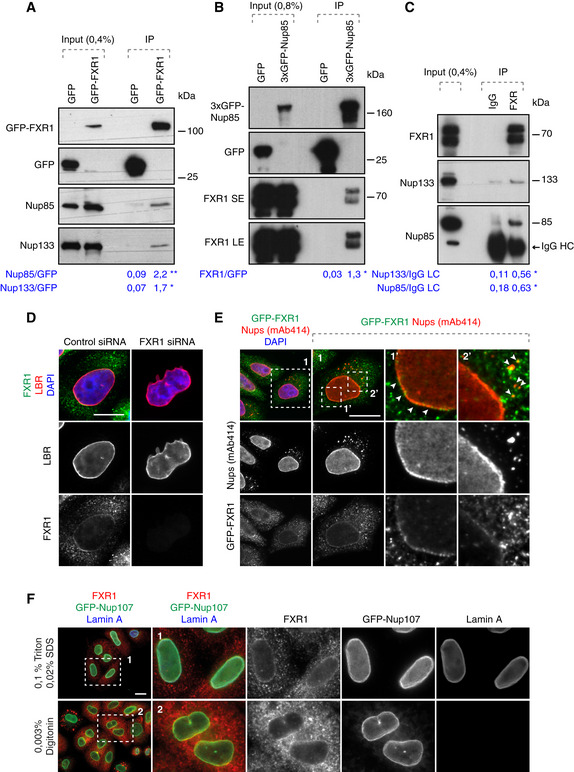
Lysates of HeLa cells stably expressing GFP alone or GFP‐FXR1 were subjected to immunoprecipitation using GFP‐Trap beads (GFP‐IP), analysed by Western blot and quantified (shown a mean value, *P < 0.05, **P < 0.01; N = 3).
Lysates of HeLa cells stably expressing GFP alone or 3xGFP‐Nup85 were immunoprecipitated using GFP‐Trap beads (GFP‐IP), analysed by Western blot and quantified (SE, short exposure, LE, long exposure; shown a mean value, *P < 0.05; N = 3).
Immunoprecipitation from HEK293T cell lysates using FXR1 antibody or IgG analysed by Western blot. The arrow points to the heavy chain of IgG (IgG HC; shown a mean value, *P < 0.05; N = 3).
HeLa cells were treated with indicated siRNAs, synchronized by double thymidine block, and released for 12 h and analysed by immunofluorescence microscopy for the lamin B receptor (LBR) to label the NE, and FXR1.
HeLa cells stably expressing GFP‐FXR1 were analysed by immunofluorescence microscopy for GFP and mAb414, which labels FG‐Nups. The magnified framed regions are shown in the corresponding numbered panels. The arrowheads indicate NE and cytoplasmic localization of GFP‐FXR1.
HeLa cells stably expressing GFP‐Nup107 were synchronized by double thymidine block and released for 12 h, permeabilized with Triton/SDS or digitonin for antibodies to access the nuclear and cytoplasmic or cytoplasmic side of the nucleus, respectively, and analysed by immunofluorescence microscopy.
Data information: Scale bars are 5 μm. Statistical significance was assessed by unpaired two‐tailed Student's
‐test.