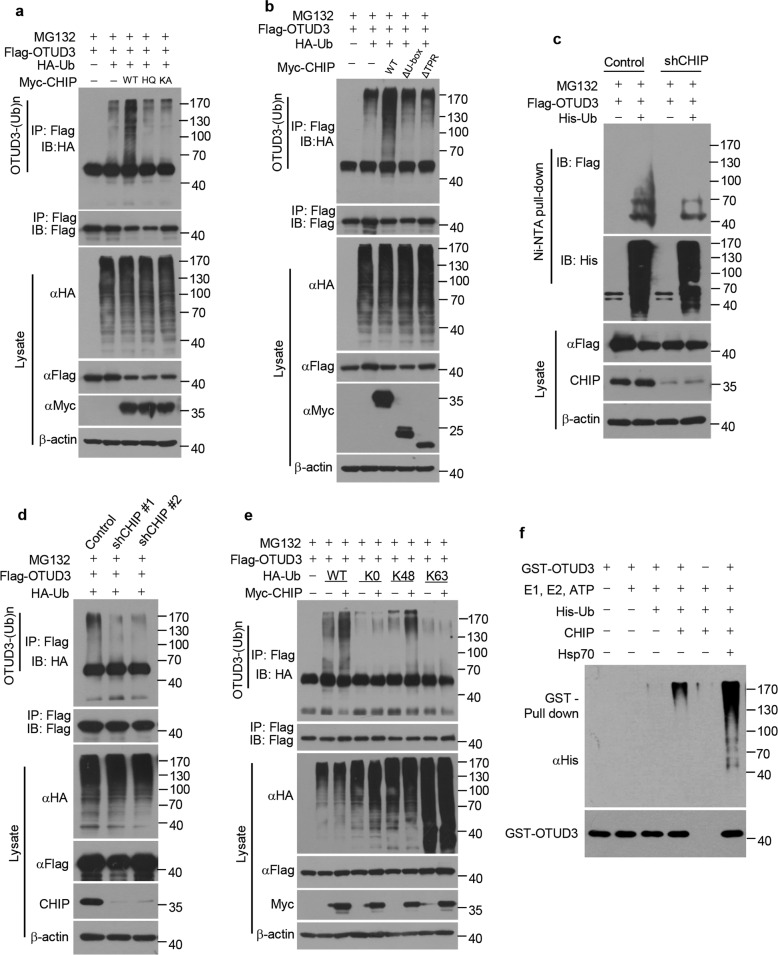
Fig. 3. CHIP promotes ubiquitylation of OTUD3.
a 293T cells were transfected for 40 h with Flag-tagged OTUD3, HA tagged Ub, Myc-tagged WT CHIP, H260Q (HQ) mutant CHIP or K30A (KA) mutant CHIP alone or in combination, and then treated for 8 h with MG132 (20 µM). Cell lysates were immunoprecipitated with Flag antibody, followed by western blotting with indicated antibodies. b 293T cells were transfected for 40 h with Flag-tagged OTUD3, HA tagged Ub, Myc-tagged WT CHIP, ΔU-box or ΔTPR alone or in combination, and then treated for 8 h with MG132 (20 µM). Cell lysates were immunoprecipitated with Flag antibody, followed by western blotting with indicated antibodies. c H1299 cells stably expressing control shRNA or CHIP shRNA were transfected for 40 h with Flag-tagged OTUD3 alone or together with His tagged Ub, and then treated for 8 h with MG132 (20 µM). Cell lysates were subjected to pull-down with Ni2+ beads, followed by western blotting with indicated antibodies. d H1299 cells stably expressing control shRNA or CHIP shRNAs (shCHIP #1, shCHIP #2) were transfected for 40 h with Flag-tagged OTUD3 and HA tagged Ub, and then treated for 8 h with MG132 (20 µM). Cell lysates were immunoprecipitated with Flag antibody, followed by western blotting with indicated antibodies. e 293T cells were transfected for 40 h with Flag-tagged OTUD3, Myc-tagged CHIP, HA tagged WT Ub or mutant Ub (K0, no Lys; K48, Lys 48-only; K63, Lys 63-only) alone or in combination, and then treated for 8 h with MG132 (20 µM). Cell lysates were immunoprecipitated with Flag antibody, followed by western blotting with indicated antibodies. f The bacteria-expressed and purified GST-tagged OTUD3 proteins were incubated with or without commercial E1, UbE2D3 (E2), ATP, CHIP (E3), Hsp70 and His tagged Ub for 2 h at 37 °C. The mixtures were subjected to GST pull-down and western blotting with indicated antibodies.