Fig. 4. Proline metabolism in the regulation of cell survial and redox balance.
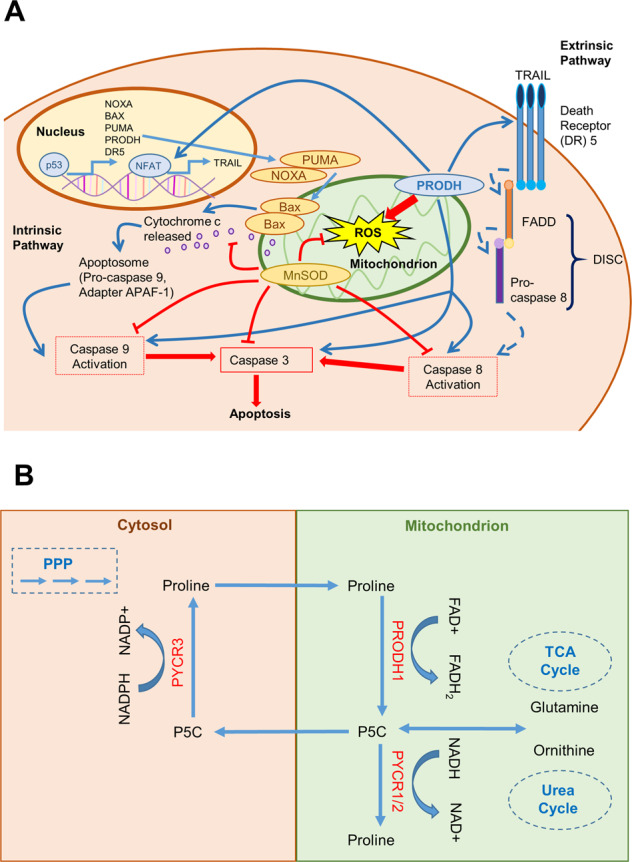
A PRODH has been implicated in the activation of apoptosis via both the intrinsic and extrinsic pathways. Activation of the intrinsic pathway by reactive oxygen species (ROS) is mediated by the p53-induced transcriptional activation of genes, including PRODH1. In the intrinsic pathway, the BH3-only proteins, such as NOXA and PUMA, inactivate the pro-survival proteins such as BCL-2 and lead to the release of pro-apoptotic proteins such as BAX. This allows the permeabilization of the mitochondrial outer membrane. Cytochrome c is released from the mitochondria and stimulates assembly of the apoptosome containing APAF-1 and pro-caspase 9. The apoptosome leads to the activation of caspase 9, which, in turn, activates the effector caspase 3 resulting in apoptosis. The extrinsic pathway of apoptosis is initiated by the activation of the death receptor of the TNF superfamily, such FAS and TRAIL. Receptor engagement and trimerization lead to the recruitment and activation of caspase-8 in the death inducing signaling complex (DISC), which in turn activates the effector caspase 3, resulting in apoptosis. PRODH1 has been shown to induce expression of the death receptor 5 (DR5) and its ligand TRAIL through stimulation of the nuclear factor of activated T cells (NFAT) transcription factor. B Schematic representation of the proposed proline cycle. L-proline is oxidized to P5C by PRODH1 in the mitochondria. P5C is then reduced back to L-proline through the action of the PYCR3 enzyme in the cytosol, a reaction coupled with the oxidation of NADPH. The cycle shuttles electrons from the cytosol to the mitochondria and stimulates flux through the PPP via oxidation of NADPH. In this way, the proline cycle can potentially contribute to nucleotide biosynthesis.
