Abstract
Oral and genital mucosal epithelia are multistratified epithelial barriers with well-developed tight and adherens junctions. These barriers serve as the first line of defense against many pathogens, including human immunodeficiency virus (HIV). HIV interaction with the surface of mucosal epithelial cells, however, may activate transforming growth factor-beta (TGF-β) and mitogen-activated protein kinase signaling pathways. When activated, these pathways may lead to the disruption of epithelial junctions and epithelial–mesenchymal transition (EMT). HIV-induced impairment of the mucosal barrier may facilitate the spread of pathogenic viral, bacterial, fungal, and other infectious agents. HIV-induced EMT promotes highly motile/migratory cells. In oral and genital mucosa, if EMT occurs within a human papillomavirus (HPV)-infected premalignant or malignant cell environment, the HPV-associated neoplastic process could be accelerated by promoting viral invasion of malignant cells. HIV also internalizes into oral and genital mucosal epithelial cells. The majority (90%) of internalized virions do not cross the epithelium, but are retained in endosomal compartments for several days. These sequestered virions are infectious. Upon interaction with activated peripheral blood mononuclear cells and CD4+ T lymphocytes, epithelial cells containing the virus can be transferred. The induction of HIV-1 release and the cell-to-cell spread of virus from epithelial cells to lymphocytes is mediated by interaction of lymphocyte receptor function-associated antigen-1 with the epithelial cell receptor intercellular adhesion molecule-1. Thus, mucosal epithelial cells may serve as a transient reservoir for HIV, which could play a critical role in viral transmission.
Keywords: endosomal sequestration, epithelial-mesenchymal transition, human immunodeficiency virus
1 |. INTRODUCTION
Human immunodeficiency virus (HIV) interaction with oral and genital mucosal epithelia may occur upon primary HIV contact and during systemic HIV/AIDS disease. Oral and genital epithelial cells may express one or more of the following proteins, which may facilitate HIV binding and entry: C-X-C chemokine receptor type 4 (CXCR4), C-C chemokine receptor type 5 (CCR5), galactosylceramide (GalCer), heparan sulfate proteoglycans (HSPG), mannose receptor, and T-cell immunoglobulin and mucin domain 1 (TIM-1) (Bobardt et al., 2007; Dwinell, Eckmann, Leopard, Varki, & Kagnoff, 1999; Herrera et al., 2016; Howell, Asin, Yeaman, & Wira, 2005; Liu et al., 2003; Tugizov et al., 2011, 2012; Yasen, Herrera, Rosbe, Lien, & Tugizov, 2018). HIV interaction with the epithelial surface may cause the disruption of tight and adherens junctions, leading to epithelial–mesenchymal transition (EMT) (Lien, Mayer, Herrera, & Rosbe, 2019). After HIV internalization into epithelial cells, virions can be sequestered in vesicular–endosomal compartments to be released and transferred into activated lymphocytes upon cell-to-cell interactions (Yasen, Herrera, Rosbe, Lien, & Tugizov, 2017; Yasen et al., 2018).
2 |. HIV-INDUCED EMT OF ORAL AND GENITAL EPITHELIAL CELLS
2.1 |. Cell-free HIV virions, HIV proteins gp120 and Tat induce EMT in oral and genital epithelial cells
HIV-1 interaction with oral and genital mucosal epithelial cells may depolarize epithelia and disrupt their tight and adherens junctions (Sufiawati & Tugizov, 2014, 2018), leading to emergence of cells with the EMT phenotype (Lien et al., 2019) (Figure 1). During embryonic development, EMT is a normal multistep epigenetic process that coordinates and regulates the differentiation of cell lineage identity (Moustakas & Heldin, 2007). The EMT phenotype, however, also plays a role in neoplastic processes, facilitating growth, migration, invasion, and metastasis of tumor cells (Moustakas & Heldin, 2007). During cancer-associated EMT, epithelial cells lose cell–cell junctions and baso-apical polarity and become apoptosis-resistant, proliferative, mobile, and invasive (Talbot, Bhattacharya, & Kuo, 2012).
F I G U R E 1.
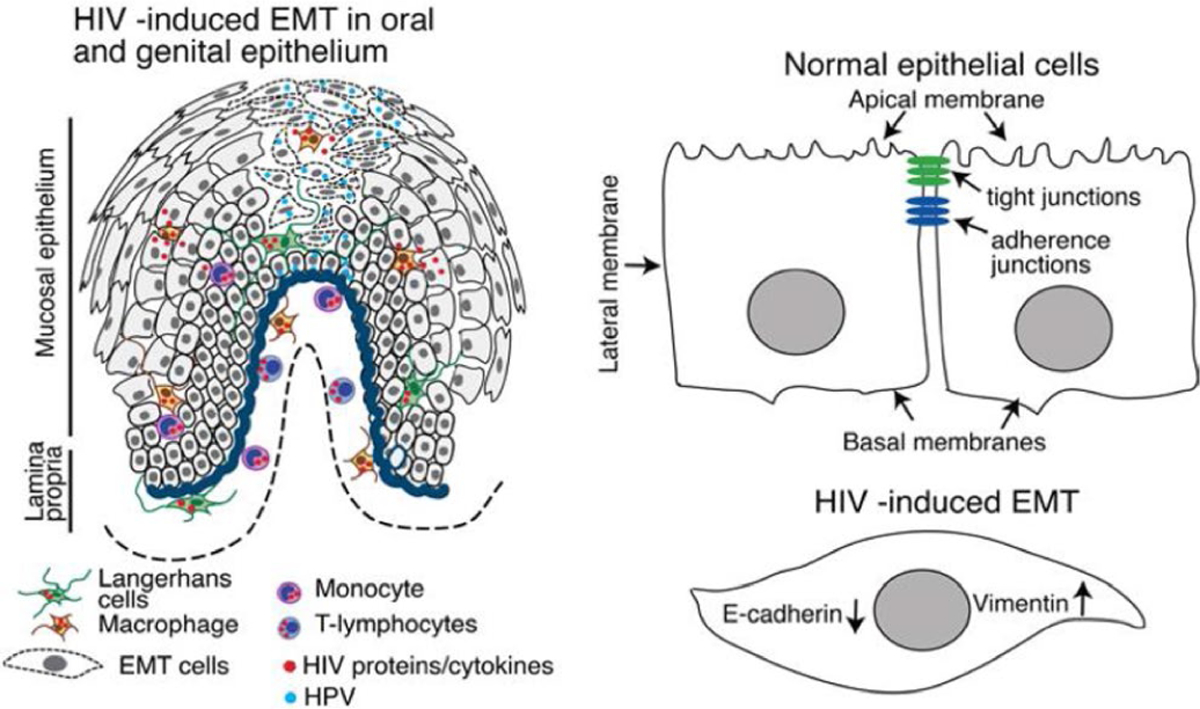
Model of HIV-mediated EMT in the progression of HPV-associated cancer. In HIV-infected individuals, HIV-infected CD4 + lymphocytes, monocyte/macrophages, and Langerhans/dendritic cells (LC/DCs) migrate into oropharyngeal and genital epithelia, secreting virions, the HIV proteins Tat and gp120, and/or cytokines, including TNF-α and IFN-γ, within the mucosal environment (A). The interaction of cell-free HIV virions and proteins/cytokines with epithelial cells activates MAPK and TGF-β, leading to the disruption of epithelial junctions and induction of the EMT phenotype (A and B). Within the HPV-associated premalignant or malignant cell environment of oral and genital mucosa, HIV-induced EMT cells are highly invasive. If EMT occurs, the neoplastic process could be accelerated by promoting the invasion of malignant cells. Inhibition of EMT or induction of MET may normalize the epithelial morphology and physiological functions. Induction of MET in the HIV/HPV-coinfected mucosal epithelia could significantly reduce the progression of HPV-associated neoplasia
Epigenetic reprogramming to facilitate EMT involves multiple signaling pathways. The dominant canonical regulatory network for cancer-associated EMT, however, is the transforming growth factor-beta (TGF-β) signaling pathway (Gordon & Blobe, 2008). TGF-β signaling is activated by binding of mature TGF-β to TGF-β-R2 leading to recruitment and activation of downstream molecules, including Smad family transcription factor complexes. These complexes activate the transcriptional regulators Snail, Slug, and Twist1, which lead to downregulation of E-cadherin transcription and upregulation of vimentin, N-cadherin, and fibronectin (Meulmeester & Ten Dijke, 2011; Wendt, Tian, & Schiemann, 2012). Upon inhibition of downregulation of E-cadherin and upregulation of vimentin, the EMT phenotype is reversible; epithelial morphology normalizes, a process known as mesenchymal–epithelial transition (MET) (Takenouchi, Yoshioka, Yamanaka, & Kitani, 2010).
The EMT phenotype in tonsil, cervical, and foreskin epithelial cells is induced upon prolonged interaction with cell-free HIV-1 virions, and viral envelope and transactivator proteins gp120 and Tat, respectively (Lien et al., 2019). Characteristic of the EMT phenotype, E-cadherin expression is reduced, while the expression of vimentin and N-cadherin is upregulated. HIV gp120- and Tat-induced EMT is mediated by activation of TGF-β and mitogen-activated protein kinase (MAPK) signaling, SMAD2 phosphorylation, and upregulation of transcription factors Slug, Snail, Twist1, and ZEB1, promoting characteristic mesenchymal cells that are highly migratory on collagen-coated membranes.
To induce HIV-1-specific EMT, the source of TGF-β may be the elevated blood levels in HIV-infected individuals (Amarnath, Dong, Li, Wu, & Chen, 2007; Elrefaei et al., 2010). TGF-β expression is upregulated by the AP-1 transcription factor (Birchenall-Roberts et al., 1990), which is induced by MAPK signaling (Glauser & Schlegel, 2007). HIV is a strong activator of MAPK signaling and two viral proteins gp120 and Tat independently activate MAPK. HIV Tat is a transactivator protein that binds to α5β1, α5β3, and αvβ3 integrins (Barillari, Sgadari, Fiorelli, et al., 1999; Barillari, Sgadari, Palladino, et al., 1999; Urbinati et al., 2005) and induces Ras-dependent activation of MAPK (Toschi et al., 2006). Viral envelope gp120 binds to the chemokine receptors CXCR4 and CCR5, also activating MAPK (Del Corno et al., 2001; Freedman, Liu, Del Corno, & Collman, 2003; Lee et al., 2003). HIV gp120 also binds to HSPG (Bobardt et al., 2007; Herrera et al., 2016; Howell et al., 2005; Liu et al., 2003; Tugizov et al., 2011, 2012), which binds TGF-β superfamily proteins, and leads to activation of TGF-β signaling (Rider & Mulloy, 2017).
HIV-1 gp120 and Tat may also activate expression of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) (Leghmari, Bennasser, Tkaczuk, & Bahraoui, 2008; Planes, Serrero, Leghmari, BenMohamed, & Bahraoui, 2018), which may also activate MAPK and TGF-β to stimulate EMT (Bates & Mercurio, 2003; Lv et al., 2015).
2.2 |. Human papillomavirus-associated orogenital cancer is significantly increased in HIV-infected individuals
The incidence of Human papillomavirus (HPV)-associated oropharyngeal cancer is about sixfold greater in HIV-infected individuals than in HIV-negative individuals (Engels et al., 2008; Grulich, van Leeuwen, Falster, & Vajdic, 2007; Powles et al., 2009). In addition to oral cancer, the incidence of HPV-associated anal and cervical cancer is 80 and 22 times greater, respectively, in HIV-infected individuals than in HIV-negative individuals (Denny et al., 2012; Mallari et al., 2012; Palefsky, 2012). These observations lead to the question of how HIV-1 predisposes to an increased incidence of HPV-associated cancers?
2.3 |. HIV-induced EMT may promote the progression of HPV-associated neoplasia
Epithelial–mesenchymal transition occurs in most epithelial cancers, including HPV-associated neoplastic processes (Hsu et al., 2007; Lee & Shen, 2012). In HPV 16-infected cervical cancers, reduction of E-cadherin and induction of vimentin expression are associated with the acquisition of the mesenchymal phenotype (Gilles et al., 1996; Lee, Chou, Tang, & Shen, 2008). Reduced E-cadherin expression and increased expression of vimentin and fibronectin appear to be driven by expression of the HPV oncoproteins E6/E7 in normal foreskin keratinocytes, which acquired a spindle-like morphology (Hellner, Mar, Fang, Quackenbush, & Munger, 2009). HPV E6/E7 may synergize with TGF-β -treated HPV 16-immortalized human cervical cancer cells (SiHa) to promote EMT and increased invasion (Yi et al., 2002).
While HIV may induce the EMT phenotype in oral and genital epithelia, HIV-induced EMT alone does not lead to malignancy. As described above, HPV E6/E7 proteins induce the EMT phenotype. Although the incidence of HPV-associated cancer is clearly increased in HIV-infected individuals, little is known about the mechanisms by which HIV potentiates the development of HPV-associated oral and anogenital cancer. EMT may be induced by both HIV and HPV, but through different mechanisms, suggesting that HIV and HPV may induce EMT additively or synergistically, with important consequences for the development of HPV-associated neoplasia in HIV-infected individuals (Figure 1).
There are two scenarios in which HIV and HPV might interact in the oral and anogenital epithelia. (a) If an individual has a prior, persisting HPV-infected epithelial lesion, subsequent acquisition of HIV infection and HIV proteins in the microenvironment might accelerate disease progression because of synergy in the induction of EMT. Conversely, HIV proteins may inhibit MET, which might otherwise have occurred, increasing the risk of disease persistence and progression. (b) If an individual acquires HIV and associated EMT followed by HPV infection, the synergistic induction of EMT by HPV E6 and E7 proteins would increase the risk of carcinoma incidence, persistence, and progression.
3 |. HIV INTERNALIZES INTO OR AL AND GENITAL EPITHELIAL CELLS TO FORM INTR A-EPITHELIAL RESERVOIRS
3.1 |. HIV endocytosis and macropinocytosis into oral and genital epithelial cells lead to viral sequestration in the vesicles
The interaction of HIV-1 with epithelial surface proteins HSPG, GalCer, and TIM-1, and internalization of virus by clathrin- and caveolin/lipid raft-associated endocytosis and macropinocytosis, leads to viral sequestration in vesicles (Yasen et al., 2017, 2018) (Figure 2). Of initially inoculated virions, about 20% attach to the cell surface and ~4% internalize into cells. About 0.01% of the initial inoculum undergoes transcytosis, indicating that >95% of internalized virions sequester in the cells (Yasen et al., 2018).
F I G U R E 2.
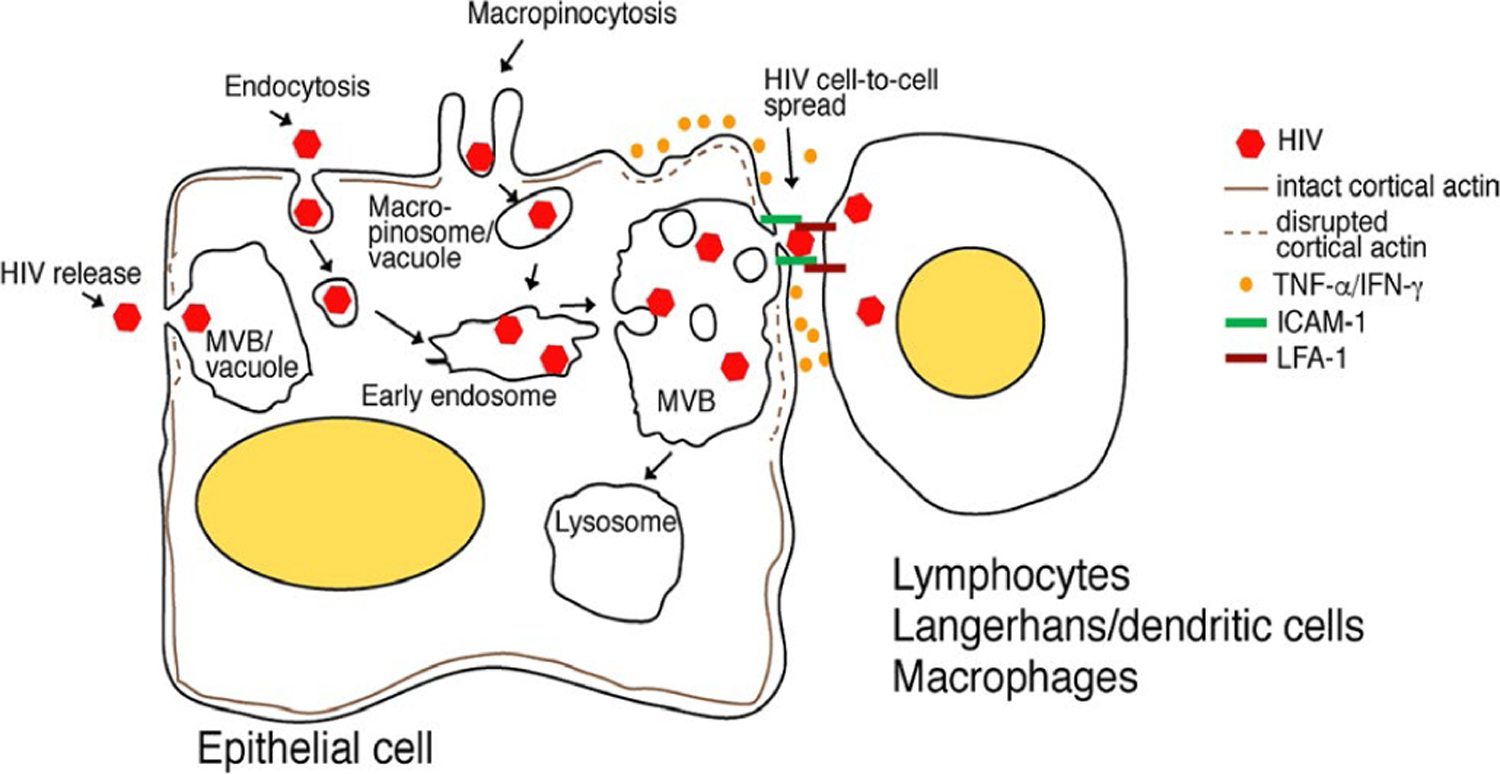
Model of HIV sequestration in oral and genital epithelia. HIV-1 internalization into oropharyngeal and genital epithelia by endocytosis and macropinocytosis subsequently delivers virus to the early endosomes, which maturate and form MVB-containing virions. Some macropinosomes containing virus may also fuse with one another and form vacuoles containing HIV. The lymphocytes, macrophages, and LC/DCs infiltrating the mucosal epithelia bind to epithelial cells through lymphocyte LFA-1 and epithelial ICAM-1. Infiltrating immune cells under activation/inflammatory conditions secrete TNF-α and IFN-γ, which induce the disruption of cortical actin, leading to the induction of HIV exocytosis. Adhesion of immune cells to epithelial cells may form a virological synapse and facilitate HIV spread from epithelia into CD4+ lymphocytes, macrophages, and LC/DCs. Inactivation of intravesicular HIV may inhibit transmucosal viral transmission
Although GalCer and HSPG are well known to contribute to HIV endocytosis, TIM-1 stimulates HIV internalization by macropinocytosis as we reported (Yasen et al., 2018). TIM-1 is a phosphatidylserine (PS) receptor; their binding induces micropinocytosis, which is an actin-dependent process induced by membrane ruffling and the formation of large vacuoles, that is, macropinosomes (Mercer & Helenius, 2009; Tugizov, Herrera, & Palefsky, 2013). The outer leaflet of the HIV-1 envelope contains PS (Aloia, Jensen, Curtain, Mobley, & Gordon, 1988; Aloia, Tian, & Jensen, 1993; Callahan et al., 2003; Gekonge, Schiralli, Schlegel, & Henderson, 2006), and binding of HIV-1-associated PS with TIM-1 of oral and genital epithelial cells facilitates viral micropinocytosis (Yasen et al., 2018).
Thus, GalCer/HSPG-mediated HIV endocytosis and TIM-1-induced micropinocytosis deliver virions into early endosomes, which subsequently mature and form multivesicular bodies (MVB), where HIV-1 becomes sequestered (Yasen et al., 2017, 2018). Virions may also sequester in vacuoles formed by homophilic fusion of macropinosomes (Araki, Hamasaki, Egami, & Hatae, 2006; Falcone et al., 2006; Hamasaki, Araki, & Hatae, 2004; Hewlett, Prescott, & Watts, 1994; Racoosin & Swanson, 1993; Schnatwinkel et al., 2004).
HIV-1 intra-epithelial sequestration without substantial HIV-1 release is common in tonsil, cervical, and foreskin epithelial cells isolated from different donors (Yasen et al., 2017, 2018), suggesting that this phenomenon may be relevant to the biological functions of both oral and genital mucosal epithelia. Although these epithelia are found at different anatomical sites, they have similar morphological features—squamous epithelial morphology and stratified organization—and serve as portals of entry for HIV-1 (Bouschbacher et al., 2008; Carias et al., 2013; Dinh et al., 2015; Tugizov et al., 2011, 2012; Zhou et al., 2011) (Kohli et al., 2014; Moyes, Islam, Kohli, & Naglik, 2016). Squamous epithelia from different anatomical locations may also have similar mechanisms for HIV-1 sequestration in their endosomal/vesicular compartments (Yasen et al., 2017, 2018).
3.2 |. Activated lymphocytes induce release and uptake of intra-epithelial virions
HIV-infected epithelial cells release of trapped virions into co-cultivated activated lymphocytes (Yasen et al., 2017, 2018). During the interaction of lymphocyte receptor function-associated antigen-1 (LFA-1) with the epithelial cell receptor intercellular adhesion molecule-1 (ICAM-1), epithelial cells depolarize and the disruption of cortical actin contributes to viral release. Furthermore, LFA-1/ICAM-1 promotes formation of virological synapses and cell-to-cell spread of HIV-1 from epithelial cells directly to activated peripheral blood mononuclear cells (PBMC) and CD4+ T lymphocytes. Thus, the ICAM-1/LFA-1-induced depolarization of epithelial cells and reorganization of the actin network are critical for (a) viral exocytosis from epithelial cells and (b) cell-to-cell viral spread from epithelial cells to lymphocytes, potentially initiating HIV mucosal transmission.
3.3 |. Inflammation of mucosal epithelium may induce the release of sequestrated HIV from endosomes of epithelial cells
We showed that proinflammatory cytokines TNF-α and IFN-γ induce the disruption of oral epithelial tight junctions (Tugizov, 2016), reorganization of cortical actin, and release of intravesicular virus from infant tonsil epithelial cells (Yasen et al., 2017), suggesting that inflammation of mucosal epithelium can induce the release of sequestered virus and its paracellular spread. Induction of HIV release from epithelial cells by activated PBMC (Yasen et al., 2017) suggests that proinflammatory cytokines secreted by activated immune cells may play a critical role in the reorganization of cortical actin and release of intravesicular virus from epithelial cells (see above). Thus, the activation of immune cells due to inflammation of the mucosal environment could be one of the critical factors for HIV mucosal transmission.
4 |. SUMMARY
Over the last several years, there have been significant advances in our understanding of the interaction of HIV with oral and genital mucosal epithelia. The two distinct features of HIV interaction with mucosal epithelial cells are as follows: (a) interaction of HIV with the mucosal surface disrupts epithelial junctions and induces the EMT phenotype and (b) internalization of HIV into epithelial cells establishes virus sequestration in the endosomes. Thus, our future goals should focus on identifying the critical molecular targets for EMT termination and MET induction. These targets could lead to the development of drugs that terminate EMT and induce MET and substantially reduce the development of HPV-associated high-grade lesions and invasive cancer. Future research should also focus on (a) investigating molecular mechanisms of HIV vesicular sequestration and (b) designing new antiviral drugs that would inhibit viral sequestration and/or inactivate endosomal virions.
ACKNOWLEDGEMENTS
This project was supported by the NIDCR R01DE028129 and NCI R01CA232887 grants (to SMT).
Footnotes
CONFLICT OF INTEREST
This paper has only one author, Sharof Tugizov, and he has no conflict of interest. He did not receive grants or speaker fees from any commercial body within the past two years.
REFERENCES
- Aloia RC, Jensen FC, Curtain CC, Mobley PW, & Gordon LM (1988). Lipid composition and fluidity of the human immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America, 85(3), 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia RC, Tian H, & Jensen FC (1993). Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proceedings of the National Academy of Sciences of the United States of America, 90(11), 5181–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarnath S, Dong L, Li J, Wu Y, & Chen W (2007). Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25- T cells. Retrovirology, 4, 57 10.1186/1742-4690-4-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Hamasaki M, Egami Y, & Hatae T (2006). Effect of 3-methyladenine on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell Structure and Function, 31(2), 145–157. 10.1247/csf.06029 [DOI] [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, … Ensoli B (1999). The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphavbeta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood, 94(2), 663–672. [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Palladino C, Gendelman R, Caputo A, Morris CB, … Ensoli B (1999). Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi’s sarcoma via induction of basic fibroblast growth factor and the alpha v beta 3 integrin. Journal of Immunology, 163(4), 1929–1935. [PubMed] [Google Scholar]
- Bates RC, & Mercurio AM (2003). Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Molecular Biology of the Cell, 14(5), 1790–1800. 10.1091/mbc.e02-09-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenall-Roberts MC, Ruscetti FW, Kasper J, Lee HD, Friedman R, Geiser A, … Kim SJ (1990). Transcriptional regulation of the transforming growth factor beta 1 promoter by v-src gene products is mediated through the AP-1 complex. Molecular and Cellular Biology, 10(9), 4978–4983. 10.1128/MCB.10.9.4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, & Gallay PA (2007). Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. Journal of Virology, 81(1), 395–405. 10.1128/JVI.01303-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouschbacher M, Bomsel M, Verronese E, Gofflo S, Ganor Y, Dezutter-Dambuyant C, & Valladeau J (2008). Early events in HIV transmission through a human reconstructed vaginal mucosa. Aids, 22(11), 1257–1266. 10.1097/QAD.0b013e3282f736f4 [DOI] [PubMed] [Google Scholar]
- Callahan MK, Popernack PM, Tsutsui S, Truong L, Schlegel RA, & Henderson AJ (2003). Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. Journal of Immunology, 170(9), 4840–4845. 10.4049/jimmunol.170.9.4840 [DOI] [PubMed] [Google Scholar]
- Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, … Hope TJ (2013). Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. Journal of Virology, 87(21), 11388–11400. 10.1128/JVI.01377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corno M, Liu QH, Schols D, de Clercq E, Gessani S, Freedman BD, & Collman RG (2001). HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood, 98(10), 2909–2916. 10.1182/blood.V98.10.2909 [DOI] [PubMed] [Google Scholar]
- Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, & Palefsky J (2012). Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine, 30(Suppl 5), F168–F174. 10.1016/j.vaccine.2012.06.045 [DOI] [PubMed] [Google Scholar]
- Dinh MH, Anderson MR, McRaven MD, Cianci GC, McCoombe SG, Kelley ZL, … Hope TJ (2015). Visualization of HIV-1 interactions with penile and foreskin epithelia: Clues for female-to-male HIV transmission. PLoS Path, 11(3), e1004729 10.1371/journal.ppat.1004729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinell MB, Eckmann L, Leopard JD, Varki NM, & Kagnoff MF (1999). Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology, 117(2), 359–367. 10.1053/gast.1999.0029900359 [DOI] [PubMed] [Google Scholar]
- Elrefaei M, Burke CM, Baker CA, Jones NG, Bousheri S, Bangsberg DR, & Cao H (2010). HIV-specific TGF-beta-positive CD4+ T cells do not express regulatory surface markers and are regulated by CTLA-4. AIDS Research and Human Retroviruses, 26(3), 329–337. 10.1089/aid.2009.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, … Goedert JJ (2008). Cancer risk in people infected with human immunodeficiency virus in the United States. International Journal of Cancer. Journal International du Cancer, 123(1), 187–194. 10.1002/ijc.23487 [DOI] [PubMed] [Google Scholar]
- Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, & Meldolesi J (2006). Macropinocytosis: Regulated coordination of endocytic and exocytic membrane traffic events. Journal of Cell Science, 119(Pt 22), 4758–4769. 10.1242/jcs.03238 [DOI] [PubMed] [Google Scholar]
- Freedman BD, Liu QH, Del Corno M, & Collman RG (2003). HIV-1 gp120 chemokine receptor-mediated signaling in human macrophages. Immunologic Research, 27(2–3), 261–276. [DOI] [PubMed] [Google Scholar]
- Gekonge BN, Schiralli G, Schlegel RA, & Henderson AJ (2006). Signal transduction induced by apoptotic cells inhibits HIV transcription in monocytes/macrophages. Journal of Leukocyte Biology, 80(4), 953–960. 10.1189/jlb.1105638 [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Piette J, Delvigne AC, Thompson EW, Foidart JM, & Birembaut P (1996). Vimentin expression in cervical carcinomas: Association with invasive and migratory potential. Journal of Pathology, 180(2), 175–180. [DOI] [PubMed] [Google Scholar]
- Glauser DA, & Schlegel W (2007). Sequential actions of ERK1/2 on the AP-1 transcription factor allow temporal integration of metabolic signals in pancreatic beta cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 21(12), 3240–3249. 10.1096/fj.06-7798com [DOI] [PubMed] [Google Scholar]
- Gordon KJ, & Blobe GC (2008). Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochimica et Biophysica Acta, 1782(4), 197–228. 10.1016/j.bbadis.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Grulich AE, van Leeuwen MT, Falster MO, & Vajdic CM (2007). Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet, 370(9581), 59–67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- Hamasaki M, Araki N, & Hatae T (2004). Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 277(2), 298–306. 10.1002/ar.a.20027 [DOI] [PubMed] [Google Scholar]
- Hellner K, Mar J, Fang F, Quackenbush J, & Munger K (2009). HPV16 E7 oncogene expression in normal human epithelial cells causes molecular changes indicative of an epithelial to mesenchymal transition. Virology, 391(1), 57–63. 10.1016/j.virol.2009.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R, Morris M, Rosbe K, Feng Z, Weinberg A, & Tugizov S (2016). Human beta-defensins 2 and −3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology, 487, 172–187. 10.1016/j.virol.2015.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, & Watts C (1994). The coated pit and macropinocytic pathways serve distinct endosome populations. Journal of Cell Biology, 124(5), 689–703. 10.1083/jcb.124.5.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AL, Asin SN, Yeaman GR, & Wira CR (2005). HIV-1 infection of the female reproductive tract. Current HIV/AIDS Reports, 2(1), 35–38. 10.1007/s11904-996-0007-0 [DOI] [PubMed] [Google Scholar]
- Hsu YM, Chen YF, Chou CY, Tang MJ, Chen JH, Wilkins RJ, … Shen MR (2007). KCl cotransporter-3 down-regulates E-cadherin/beta-catenin complex to promote epithelial-mesenchymal transition. Cancer Research, 67(22), 11064–11073. 10.1158/0008-5472.CAN-07-2443 [DOI] [PubMed] [Google Scholar]
- Kohli A, Islam A, Moyes DL, Murciano C, Shen C, Challacombe SJ, & Naglik JR (2014). Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected. PLoS ONE, 9(5), e98077 10.1371/journal.pone.0098077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, & Collman RG (2003). Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. Journal of Leukocyte Biology, 74(5), 676–682. 10.1189/jlb.0503206 [DOI] [PubMed] [Google Scholar]
- Lee MY, Chou CY, Tang MJ, & Shen MR (2008). Epithelial-mesenchymal transition in cervical cancer: Correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 14(15), 4743–4750. 10.1158/1078-0432.CCR-08-0234 [DOI] [PubMed] [Google Scholar]
- Lee MY, & Shen MR (2012). Epithelial-mesenchymal transition in cervical carcinoma. American Journal of Translational Research, 4(1), 1–13. [PMC free article] [PubMed] [Google Scholar]
- Leghmari K, Bennasser Y, Tkaczuk J, & Bahraoui E (2008). HIV-1 Tat protein induces IL-10 production by an alternative TNF-alpha-independent pathway in monocytes: Role of PKC-delta and p38 MAP kinase. Cellular Immunology, 253(1–2), 45–53. 10.1016/j.cellimm.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Lien K, Mayer W, Herrera R, Rosbe K, & Tugizov SM (2019). HIV-1 proteins gp120 and tat induce the epithelial–mesenchymal transition in oral and genital mucosal epithelial cells. PLoS ONE, 14(12), e0226343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zha J, Chen H, Nishitani J, Camargo P, Cole SW, & Zack JA (2003). Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. Journal of Virology, 77(6), 3470–3476. 10.1128/JVI.77.6.3470-3476.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv N, Gao Y, Guan H, Wu D, Ding S, Teng W, & Shan Z (2015). Inflammatory mediators, tumor necrosis factor-alpha and interferon-gamma, induce EMT in human PTC cell lines. Oncology Letters, 10(4), 2591–2597. 10.3892/ol.2015.3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallari AO, Schwartz TM, Luque AE, Polashenski PS, Rauh SM, & Corales RB (2012). Anal cancer screening in HIV-infected patients: Is it time to screen them all? Diseases of the Colon and Rectum, 55(12), 1244–1250. 10.1097/DCR.0b013e31826ab4fb [DOI] [PubMed] [Google Scholar]
- Mercer J, & Helenius A (2009). Virus entry by macropinocytosis. Nature Cell Biology, 11(5), 510–520. 10.1038/ncb0509-510 [DOI] [PubMed] [Google Scholar]
- Meulmeester E, & Ten Dijke P (2011). The dynamic roles of TGF-beta in cancer. Journal of Pathology, 223(2), 205–218. 10.1002/path.2785 [DOI] [PubMed] [Google Scholar]
- Moustakas A, & Heldin CH (2007). Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Science, 98(10), 1512–1520. 10.1111/j.1349-7006.2007.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes DL, Islam A, Kohli A, & Naglik JR (2016). Oral epithelial cells and their interactions with HIV-1. Oral Diseases, 22(Suppl 1), 66–72. 10.1111/odi.12410 [DOI] [PubMed] [Google Scholar]
- Palefsky JM (2012). Antiretroviral therapy and anal cancer: The good, the bad, and the unknown. Sexually Transmitted Diseases, 39(7), 501–503. 10.1097/OLQ.0b013e31825f7921 [DOI] [PubMed] [Google Scholar]
- Planes R, Serrero M, Leghmari K, BenMohamed L, & Bahraoui E (2018). HIV-1 envelope glycoproteins induce the production of TNF-alpha and IL-10 in human monocytes by activating calcium pathway. Scientific Reports, 8(1), 17215 10.1038/s41598-018-35478-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, … Bower M (2009). Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 27(6), 884–890. 10.1200/JCO.2008.19.6626 [DOI] [PubMed] [Google Scholar]
- Racoosin EL, & Swanson JA (1993). Macropinosome maturation and fusion with tubular lysosomes in macrophages. Journal of Cell Biology, 121(5), 1011–1020. 10.1083/jcb.121.5.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CC, & Mulloy B (2017). Heparin, heparan sulphate and the TGF-beta cytokine superfamily. Molecules, 22(5), 713 10.3390/molecules22050713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, & Zerial M (2004). The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biology, 2(9), E261 10.1371/journal.pbio.0020261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufiawati I, & Tugizov SM (2014). HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PLoS ONE, 9(2), e88803 10.1371/journal.pone.0088803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufiawati I, & Tugizov SM (2018). HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. Journal of General Virology, 99(7), 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Yoshioka M, Yamanaka N, & Kitani H (2010). Reversible conversion of epithelial and mesenchymal phenotypes in SV40 large T antigen-immortalized rat liver cell lines. Cell Biology International Reports, 17(1), e00001 10.1042/CBR20100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LJ, Bhattacharya SD, & Kuo PC (2012). Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. International Journal of Biochemistry and Molecular Biology, 3(2), 117–136. [PMC free article] [PubMed] [Google Scholar]
- Toschi E, Bacigalupo I, Strippoli R, Chiozzini C, Cereseto A, Falchi M, … Ensoli B (2006). HIV-1 Tat regulates endothelial cell cycle progression via activation of the Ras/ERK MAPK signaling pathway. Molecular Biology of the Cell, 17(4), 1985–1994. 10.1091/mbc.e05-08-0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov S (2016). Human immunodeficiency virus-associated disruption of mucosal barriers and its role in HIV transmission and pathogenesis of HIV/AIDS disease. Tissue Barriers, 4(3), e1159276 10.1080/21688370.2016.1159276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, & Palefsky JM (2013). Epstein-barr virus transcytosis through polarized oral epithelial cells. Journal of Virology, 87(14), 8179–8194. 10.1128/JVI.00443-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, … Palefsky JM (2011). HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology, 409(2), 211–222. 10.1016/j.virol.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Veluppillai P, Greenspan D, Soros V, Greene WC, … Palefsky JM (2012). Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. Journal of Virology, 86(5), 2556–2570. 10.1128/JVI.06578-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbinati C, Mitola S, Tanghetti E, Kumar C, Waltenberger J, Ribatti D, … Rusnati M (2005). Integrin alphavbeta3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(11), 2315–2320. [DOI] [PubMed] [Google Scholar]
- Wendt MK, Tian M, & Schiemann WP (2012). Deconstructing the mechanisms and consequences of TGF-beta-induced EMT during cancer progression. Cell and Tissue Research, 347(1), 85–101. 10.1007/s00441-011-1199-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen A, Herrera R, Rosbe K, Lien K, & Tugizov SM (2017). Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS Path, 13(2), e1006247 10.1371/journal.ppat.1006247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen A, Herrera R, Rosbe K, Lien K, & Tugizov SM (2018). HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology, 515, 92–107. 10.1016/j.virol.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JY, Hur KC, Lee E, Jin YJ, Arteaga CL, & Son YS (2002). TGFbeta1 -mediated epithelial to mesenchymal transition is accompanied by invasion in the SiHa cell line. European Journal of Cell Biology, 81(8), 457–468. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barry de Longchamps N, Schmitt A, Zerbib M, Vacher-Lavenu MC, Bomsel M, & Ganor Y (2011). HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. PLoS Path, 7(6), e1002100 10.1371/journal.ppat.1002100 [DOI] [PMC free article] [PubMed] [Google Scholar]


