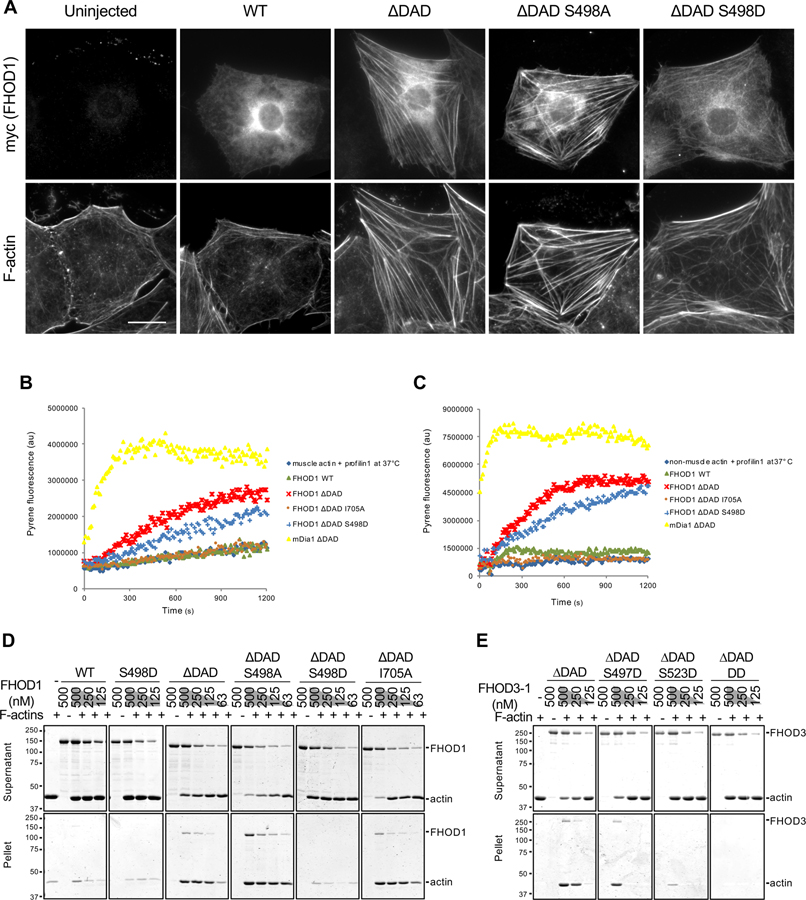
Figure 7. Effect of FHOD phosphorylation site mutants on actin binding, polymerization and bundling activities.
(A) Images of serum-starved wound-edge NIH3T3 fibroblasts expressing the indicated myc-FHOD1 mutants and stained for F-actin and myc. Bar, 20 μm. (B) Actin polymerization activity of FHOD1 mutants examined using an actin pyrene-assay. Fluorescence of pyrene-rabbit skeletal muscle actin (2 μM, 10% pyrene-labeled) pre-incubated with 4 μM human profilin1 and then incubated with 200 nM of the indicated FHOD1 mutants or 20 nM mDia1 ∆DAD at 37°C. (C) Actin polymerization activity of FHOD1 mutants examined using an actin pyrene-assay. Fluorescence of pyrene-non-muscle actin (4 μM, 10% pyrene-labeled) pre-incubated with 8 μM profilin1 and then incubated with 400 nM of various FHOD1 proteins or 40 nM mDia1 ∆DAD at 37°C. (D) Actin bundling activity of FHOD1 mutants examined using a low-speed (16,000 x g) actin co-sedimentation assay. Coomassie blue-stained SDS-polyacrylamide gels of proteins in the supernatant and pellet are shown. (E) Actin bundling activity of FHOD3-1 mutants as in D. FHOD3-1 ∆DAD DD is FHOD3-1 ∆DAD S497D S523D. In D and E, migration of molecular mass standards (kDa) is indicated at the left of each gel or blot. See also Figures S6 and S7.