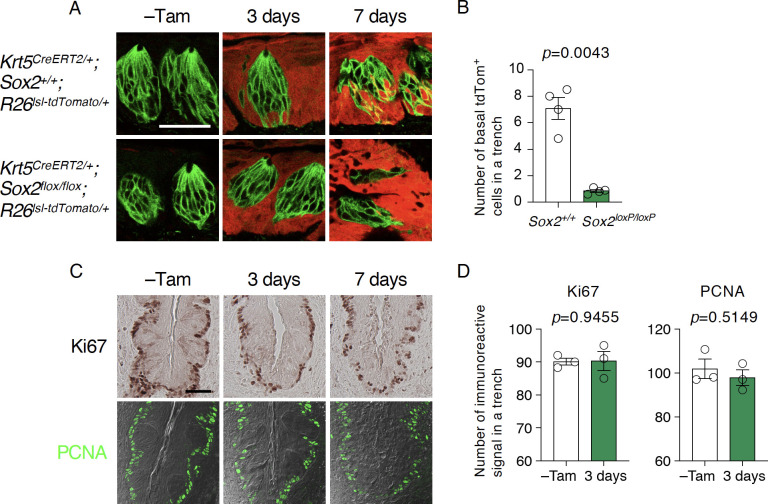
Fig 4. Sox2-deleted stem/progenitor cells are incapable of supplying new cells in taste buds.
A: Lineage tracing of epithelial stem/progenitor cells in CvP of wild type (top) and Sox2-deleted mice (bottom). Sox2 deletion was induced concominantly with the induction of tdTomato expression by tamoxifen into Krt5CreERT2/+; Sox2flox/flox; Rosa26lsl-Tom/+ mice. Taste buds were identified by KCNQ1 immunoreactivity (green). Sox2 wild type mice,–Tam (n = 1), 3 days (n = 4), and 7 days (n = 2); Sox2-deleted mice,–Tam (control: no tamoxifen injection, n = 2), 3 days (n = 4), and 7 days (n = 2). B: Quantitative analyses of newly generated basal taste bud cells from wild type (Krt5CreERT2/+; Sox2+/+; Rosa26lsl-Tom/+) and Sox2-deleted (Krt5CreERT2/+; Sox2LoxP/LoxP (cKO); Rosa26lsl-Tom/+) epithelial stem/progenitor cells. tdTomato+ basal cells in taste buds per trench were analyzed using Welch’s t-test to evaluate if the difference is statistically significant (n = 4). C: Immunohistochemical detection of Ki67 (top) and PCNA (bottom) in CvP of Krt5CreERT2/+; Sox2flox/flox mice with and without tamoxifen injection (–Tam, control). D: Quantitative analyses of immunoreactive signals to Ki67 (left) and PCNA (right) in CvP trench of Krt5CreERT2/+; Sox2flox/flox mice 3 days after tamoxifen injection and without tamoxifen injection (–Tam, control). Numbers of immunoreactive singals to proliferative cell markers per trench were statistically analyzed using Welch’s t-test (n = 3). Scale bars, 50 μm.