Abstract
Chronic exposure to noise is a detrimental environmental factor that can contribute to occupational noise-induced deafness (ONID) in industrial workers. ONID is caused by both environmental and genetic factors, and negatively impacts workers and manufacturing industries in China. Polymorphisms in the paraoxonase 2 gene (PON2) is associated with noise-induced hearing loss, and PON3 expression may modulate oxidative stress in cells and tissues by reducing the levels of reactive oxygen species, which are prominent in ONID. We conducted a matched case-control study to investigate whether PON3 polymorphisms and activity were associated with susceptibility to ONID. We genotyped PON3 single nucleotide polymorphisms (SNPs) using Sanger sequencing and measured the plasma PON3 activity using enzyme-linked immunosorbent assay. Conditional logistic regression models were fitted to evaluate the potential risk factors of ONID. A total of 300 subjects were included (n = 150 ONID and n = 150 control cases) from October 2017 to October 2019. We identified two types of genotypes for the PON3 SNPs. The independent risk factors for ONID were genotype CT and allele C with Odd’s ratio (OR) = 2.12 (95% confidence interval [CI]: 1.18–3.84) and OR = 1.68 (95% CI: 1.06–2.66) for SNP rs11767787; AG and allele A with OR = 2.09 (95% CI: 1.25–3.47) and OR = 1.87 (95% CI: 1.19–2.93) for SNP rs13226149; and CT and allele T with OR = 2.59 (95% CI: 1.44–4.67) and OR = 1.95 (95% CI: 1.22–3.14) for SNP rs17882539, respectively. Furthermore, the plasma PON3 level (> 1504 U/L) was observed to be a protective factor associated with the lowest level of ONID (less than 991 U/L) after adjusting for confounding factors (OR = 0.27, 95% CI: 0.13–0.54). In conclusion, the PON3 polymorphisms rs11767787, rs13226149, and rs17882539 and plasma PON3 activity are associated with susceptibility to ONID in the Chinese population.
Introduction
Noise is the most important environmental factor that may be detrimental to health [1, 2], especially for hearing loss [3]. Occupational noise is the most frequent occupational hazard, and the rate of disability-adjusted life years attributed to this form of noise has steadily increased worldwide from 1990 (61.11 /100 thousand) to 2017 (78.21/100 thousand) [4]. Noise-induced hearing loss (NIHL) is a sensorineural hearing deficit that begins with chronic exposure to the higher frequencies (3 to 6 kHz) and is the primary occupational disease predominantly found among industrial workers [1].
NIHL is a complex disease caused by the interaction between environmental factors and susceptibility genes [5]. The development of NIHL is mainly due to the duration of exposure, and the intensity and frequency of the noise, which results in cochlear epithelium damage. When industrial workers are exposed to excessive noise [8h ≥ 85 dB(A)] in the workplace, the cochlea consumes a lot of energy and then releases a large number of free radicals (reactive oxygen species and reactive nitrogen) locally [6]. As the antioxidant system is unable to neutralize these free radicals, the cochlear sensorial epithelium becomes damaged [7]. Consequently, genes involved in the regulation of reactive oxygen species, such as superoxide dismutase, glutathione S-transferase, and catalase, may affect the vulnerability of the cochlea to NIHL [8, 9].
Paraoxonases (PONs) are an aromatic esterase family that hydrolyze phosphate bond and degrade organophosphate compounds, aromatic carboxylates, and carbamates. The PON gene family of enzymes, which consists of PON1, PON2, and PON3, share approximately 60% and 70% identity at the amino acid, and nucleotide levels, respectively, in humans. PONs are acute phase proteins capable of degrading lipid peroxides, share considerable sequence identity, and span ~150 kb in tandem on the long arm of the human chromosome, 7q22.3−q22.1 [10, 11]. PON exerts antioxidant activity and protects against diseases such as hypertension, atherosclerosis, Alzheimer’s dementia, and Parkinson’s disease [3, 12–14]. Polymorphisms in the PON2 gene are associated with susceptibility to NIHL in Chinese industrial workers [15, 16], and may have an interaction enhancement effect with noise exposure level and other factors [17].
PON3 is the last member of the PON family of proteins to be described and is the least characterized. PON3 is a 40-kDa glycoprotein that is synthesized in the liver in a calcium-dependent manner. It has limited aromatic esterase activity and retains lipo-lactonases and N-acyl-homoserine lactone activities but cannot hydrolyze organophosphate [18]. Like PON1, PON3 is tightly bound to high-density lipoproteins in circulation, which enhance its antiatherosclerotic properties [19]. PON3 has a higher catalytic activity for statin lactones than PON1 [8, 20]. PON3 also appears to modulate oxidative stress, similar to PON1 and PON2, in cells and tissues by reducing reactive oxygen species. Previous studies have indicated that PON3 is associated with coronary artery disease, atherosclerosis, chronic liver disease, and obesity [21–23]. Liu recommended that clinicians and patients be aware of the risk of atorvastatin-associated tinnitus and permanent hearing loss based on a case report [24]. PON3 is the only enzyme that catalyzes the hydrolysis of a statin lactone ring, and a tightly linked group of PON3 polymorphisms (rs11767787, rs13226149, and rs17882539) are thought to be associated with changes in the atorvastatin δ-lactone hydrolysis [25]. Thus, we hypothesized that genetic variation of the PON3 gene and PON3 activity may play an active role in increasing the susceptibility to NIHL.
Occupational noise-induced deafness (ONID) is the most serious level of NIHL and is regarded as an important element that influences the quality of social, familial, and professional life [17]. Shenzhen, located in the south of China with a 100% urbanization rate, is an international comprehensive transportation hub and had a permanent population of 1302.66 (10 000 persons) in 2018 [26]. There are more than 8000 industrial enterprises with noise hazards in Shenzhen, and approximately190 thousand workers were at risk of occupational noise in 2018. The ONID caused by long-term occupational exposure to noise has been the first occupational disease in Shenzhen for many years.
In light of the evidence that the allele frequencies for the known PON2 polymorphisms are associated with NIHL and starting from the hypothesis that variation in the PON3 gene may play an important role in human ONID development. We aimed to identify the associations between PON3 polymorphisms and PON3 activity and susceptibility to ONID after long-term occupational exposure to noise. The outcomes obtained from the current investigation are expected to help screen individuals sensitive to noise. We hope our findings help reduce the occurrence of ONID and improve working conditions in Shenzhen, China.
Materials and methods
Study population
We focused on ONID cases in Shenzhen industrial workers. We conducted a matched case-control study to investigate the potential relationship between ONID and PON3 polymorphisms between October 1, 2017 and October 31, 2019 in Shenzhen. All participants were Han Chinese employees who were recruited from manufacturing units and industries that were noise prone such as mechanical tools, domestic appliance production and transportation, building and steel construction, respectively. All these workers were commonly exposed to a steady stream of noise [LEX, 8h ≥ 85 dB(A)] with at least three years of exposure to work-related noise. Workers with hypertension, diabetes, hyperlipidemia, cardiovascular events, history of head injury, family history of deafness, ear infection, ontological disease, drug-induced deafness, and those with other obvious causes of hearing loss (such as high temperature, explosives, heavy metals, and organic solvents) were excluded from the study. ONID cases were newly diagnosed at the Shenzhen Prevention and Treatment Center for Occupational Diseases according to the diagnosis of occupational noise-induced deafness (GBZ 49–2014) [27]. The gender, age (±2 years) and exposure time (±1 year) matched controls, consisted of healthy employees from the same company who were seeking health checkups from the Shenzhen Prevention and Treatment Center for Occupational Diseases at the same time.
Covariate assessment
A standardized, structured questionnaire was used to collect the potential occupational and risk factors for hearing loss of participants. All of them were interviewed face-to-face by trained medical workers. The questionnaire covered four types of information, including sociodemographic characteristics (age, gender, height, weight and hereditary factors), diseases (previous and present medical conditions, pharmaceutical preparations and history of head injury), participants lifestyles (smoking and drinking status), and occupation-related factors (type of work, noise exposure time for work, and leisure). The self-reported diseases were verified by specialists at the Shenzhen Prevention and Treatment Center for Occupational Diseases according to recognized international standards. The subjective effects such as tinnitus, vertigo and sensation of fullness in the ears were investigated. Smoking and drinking statuses were defined by subjects who had a minimum of one cigarette per day for at least one year and drank 50 g of wine or a bottle of beer per day for at least one year, respectively [15].
Noise intensity
We used a noise statistical analyzer (Quest SoundPro, Quest Inc., USA) to measure the intensity of noise in the workplace during the working time. As recommended by the Occupational Health Standard of the People’s Republic of China: Measurement of physical agents in the workplace—part 8: Noise [GBZ/T 189.8–2007] [28], the noise statistical analyzer was set at the ear height (about 1.5 m in the standing position and 1.1 m at the seat), with a microphone in the direction of the noise source. Each location was measured three times, and the noise exposure level for individuals was averaged. If the participants left the original noise-exposed environment, the data of occupational risk factors in previous workplaces were supported by a previous employer.
Hearing threshold
The pure-tone thresholds for each ear of all the participants were obtained using a GIS-61 audiometer (Grason-Stadler, Eden Prairie, MN) with a TDH-50P headphone (Grason-Stadler, Eden Prairie, MN) at six audiometric frequencies (0.5, 1, 2, 3, 4, and 6 kHz). Audiometry was conducted in a soundproof room by a trained technician and the background sound level was less than 30 dB(A). Hearing thresholds were adjusted by age and sex according to the Occupational Health Standard of the People’s Republic of China: Acoustics-statistical distribution of hearing thresholds as a function of age [GB/T 7582–2004] and the diagnosis of occupational noise-induced deafness (GBZ 49–2014) [27]. The hearing thresholds were classified into three types: mild [hearing threshold: 26–40 dB(A)], moderate [hearing threshold: 41–55 dB(A)] and severe [hearing threshold: ≥ 56 dB(A)].
SNP genotyping
According to Stephan et al., we selected the PON3 single nucleotide polymorphisms (SNPs) rs11767787, rs13226149, and rs17882539 to be genotyped by Sanger sequencing [25]. All ONID cases and controls donated 5 mL of fasting peripheral venous blood samples using EDTA-K2 anticoagulation tubes in the morning. Ezup column-based genomic blood DNA extraction kit (Sangon Biotech, China) was used to extract genomic DNA according to the manufacturer’s protocols [29]. A UV-Vis spectrophotometer (SMA4000; Merinton, China) and 1% agarose gel electrophoresis (AGE) (FR-980A; Shanghai Furi Technology Co., LTD, China) were used to test the quality of DNA. The primers (Table 1) for the three PON3 SNPs (rs11767787, rs13226149, and rs17882539) were designed using the Primer Premier 5 software. Polymerase chain reaction (PCR) amplification (Verity 96well; Applied Biosystems, USA) was performed using the same conditions for three PON3 SNPs: 5 min at 95°C, followed by 38 cycles of denaturation for 30 s at 94°C, annealing for 30 s at 58°C, and extension for 60 s at 72°C, with a final extension at 72°C for 10 min. The fragment of PCR products was tested using 1% agarose gel electrophoresis (AGE). The then PON3 SNPs (rs11767787, rs13226149, and rs17882539) were then genotyped using a 3730XL sequencer (Applied Biosystems, USA). As a quality control, 5% of the randomly selected samples were genotyped as blinded duplicates, and the results were 100% concordant.
Table 1. Primer sequence and characteristics for PON3 SNPs.
| SNP | Sequence (5'-3') | Genomic position | Region | L(bp) | Base Change |
|---|---|---|---|---|---|
| rs11767787 | Forward: GAGTTCGGGCAAAGTAGCAC | 3935 | Promoter | 546 | A/G |
| Reverse: CTCTGCTTGCACTCTTTTAACATTA | |||||
| rs13226149 | Forward: CCAAGCAGAATGTTGAGGGC | 5088 | Exonic | 705 | C/T |
| Reverse: GTAGGATTTCGCGGGTGTTA | |||||
| rs17882539 | Forward: TGACCTACAGCAAGCCACA | 4280 | Promoter | 877 | C/T |
| Reverse: AACACCCGCGAAATCCTA |
SNP, single nucleotide polymorphism; L, length of amplicons.
Measurement of plasma PON3 activity
Peripheral venous blood samples were centrifuged at 2500 rpm for 20 min, and the supernatant was collected to determine human plasma PON3 activity using an enzyme-linked immunosorbent assay Kit (96T; Sangon Biotech, China) according to the manufacturer’s protocols. Absorbance readings were measured at 450 nm using a microplate reader (RT-6100; Rayto, USA) with a blank hole as a reference. The results were then converted into concentrations by comparison with standard curve values. All measurements were performed in duplicate and expressed as U/L (1U = 1 mol of paraoxon hydrolyzed per minute). The correlation coefficient between the samples and the expected concentration was above 0.99, and the coefficient of variation within batches and between batches was less than 10% and 15%, respectively.
Sample size
We used two correlated (paired) proportions to calculate the sample size of this matched case-control study using the PASS 15 software. For each case patient, a matching sample of one control was also obtained. The probability of exposure (CT+TT for rs11767787; AA+AG for rs13226149 and CT+TT for rs17882539) among the control population was 0.20, and the correlation coefficient for exposure between ONID case and control population is 0.25. The power was 90% and the odds ratio (OR) was 2.00 with a = 0.05. The calculated sample size was 130 for each group; we amplified 15% of the sample size considering the refusal, and obtained a sample size of 150 ONID patients and 150 controls.
Ethical approval
The analysis presented in this manuscript is based on data and specimens in accordance with the guidelines of the Declaration of Helsinki, and all identified individual information was anonymized. This project was approved by the Ethics Committee of Shenzhen Prevention and Treatment Center for Occupational Diseases. All participants in this study signed an informed consent form before participating.
Statistical analysis
The Shapiro–Wilk test was used to check the normality of distribution of continuous variables, and mean ± standard deviation (SD) or median (interquartile range, IQR) were presented according to whether they were normally distributed. The Paired-samples t-test or Wilcoxon signed ranks test was used to detect significant differences between the ONID and control groups for continuous variables. Categorical variables were described as frequencies and percentages. The McNemar-Bowker test was used to compare the differences between the ONID and control groups. The chi-square test was used to check for deviations from the Hardy-Weinberg equilibrium (HWE) for each SNP in the control group. Conditional logistic regression models taking the matching into account were fitted to evaluate the potential relationship between PON3 polymorphisms and the risk of ONID. The multivariate analyses were adjusted for covariates (age, smoking, drinking, exposure time, and noise exposure level) with crude and adjusted ORs with their respective 95% confidence intervals (95% CIs). Furthermore, the association of genotypes with ONID was also evaluated by assuming allele models. ORs and their 95% CIs were also calculated by including the exposure plasma PNO3 activity, which was categorized into quintiles (less than P25, P25-P50, P50-P75, greater than P75), considering the lowest quintile as the reference. Statistical significance was set at p<0.05. All analyses were performed with R software, version 3.5.1.
Results
Characteristics of the participants
A total of 300 subjects were included (n = 150 ONID and n = 150 control cases) from October 2017 to October 2019. All the participants were mainly from Pingshan, Baoan, and Longgang districts, where the manufacturing industry is clustered. Of the 150 ONID cases, 139 (92.67%) and 11 (7.33%) were mild and moderate, respectively. There were no severe ONID cases in this study. The mean age was 44.37±7.55 years (range, 23–61 years) and 94% were male. The median exposure time was 11.42 (7.75) years and the intensity of noise was from 85 to 113 dB(A), with a mean noise exposure level of 93.11±5.99 dB(A). Furthermore, 34.67% and 41.67% of the study population were smoking and drinking, respectively, and 87.33% of them worked in manufacturing. There were no statistical differences between the ONID and control groups regarding age, gender, smoking, drinking, exposure time, and noise exposure level (Table 2).
Table 2. Demographics of ONID cases and control population.
| Variables | Total (n = 300) | ONID group (n = 150) | Control group(n = 150) | P value |
|---|---|---|---|---|
| Age, years | 44.37±7.55 | 44.34±7.53 | 44.41±7.59 | 0.565 |
| Gender, n (%) | 1.000 | |||
| Male | 282(94) | 141(94) | 141(94) | |
| Female | 18(6) | 9(6) | 9(6) | |
| BMI (kg/m2) | 21.59±1.20 | 21.67±1.16 | 21.51±1.23 | 0.246 |
| Smoking, n (%) | 0.659 | |||
| No | 196(65.33) | 96(64) | 100(66.67) | |
| Yes | 104(34.67) | 54(36) | 50(33.33) | |
| Drinking, n (%) | 0.569 | |||
| No | 175(58.33) | 90(60) | 85(56.67) | |
| Yes | 125(41.67) | 60(40) | 65(43.33) | |
| Type of work, n (%) | 1.000 | |||
| Manufacturing | 262(87.33) | 131(87.33) | 131(87.33) | |
| Construction | 8(2.67) | 4(2.67) | 4(2.67) | |
| Transportation | 18(6) | 9(6) | 9(6) | |
| Others | 12(4) | 6(4) | 6(4) | |
| Exposure time, years, M (IQR) | 11.42(7.75) | 11.29(7.69) | 11.54(8) | 0.363 |
| Noise Exposure Level, Db (A) | 93.11±5.99 | 93.09±5.70 | 93.12±6.30 | 0.965 |
ONID, occupational noise-induced deafness; M, median; IQR, interquartile range.
Hearing thresholds
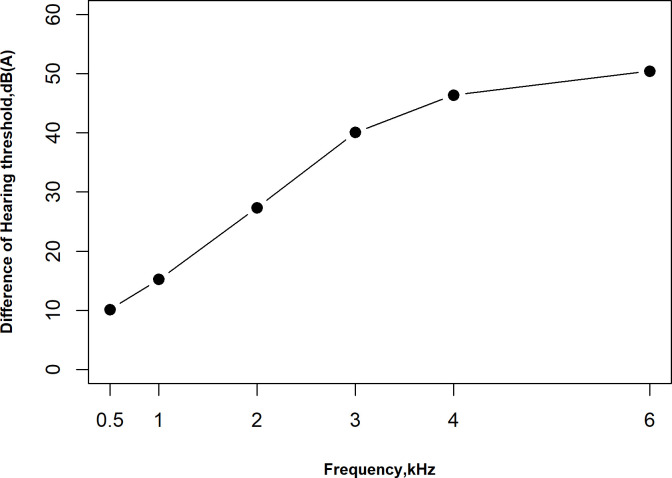
The distribution of mean hearing thresholds at frequencies from 0.5 to 6.0 kHz are shown in Table 3. The mean hearing thresholds increased as the frequency increased in both groups, and hearing thresholds were significantly higher in the ONID group than in the control group at all frequencies. The difference in hearing thresholds between the ONID and control groups also increased as the frequency increased (Fig 1).
Table 3. The distribution of hearing thresholds at different frequencies in the ONID and control groups.
| Groups | 0.5 kHz | 1.0 kHz | 2.0 kHz | 3.0 kHz | 4.0 kHz | 6.0 kHz |
|---|---|---|---|---|---|---|
| ONID group (n = 150) | 24.20±7.97 | 30.82±9.11 | 42.93±12.80 | 56.60±13.33 | 63.57±13.74 | 67.97±16.41 |
| Control group (n = 150) | 14.07±5.78 | 15.60±5.05 | 15.57±5.26 | 16.53±5.37 | 17.17±4.86 | 17.53±4.63 |
| t | 12.229 | 17.450 | 23.549 | 34.412 | 38.774 | 37.080 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
ONID, occupational noise-induced deafness.
Fig 1. Mean differences in hearing thresholds between ONID and control groups at all frequencies.
The differences of hearing thresholds between ONID and control group at frequency 3.0, 4.0 and 6.0 kHz were observed to be stable. Data are expressed as mean difference.
Fragment of PCR amplification
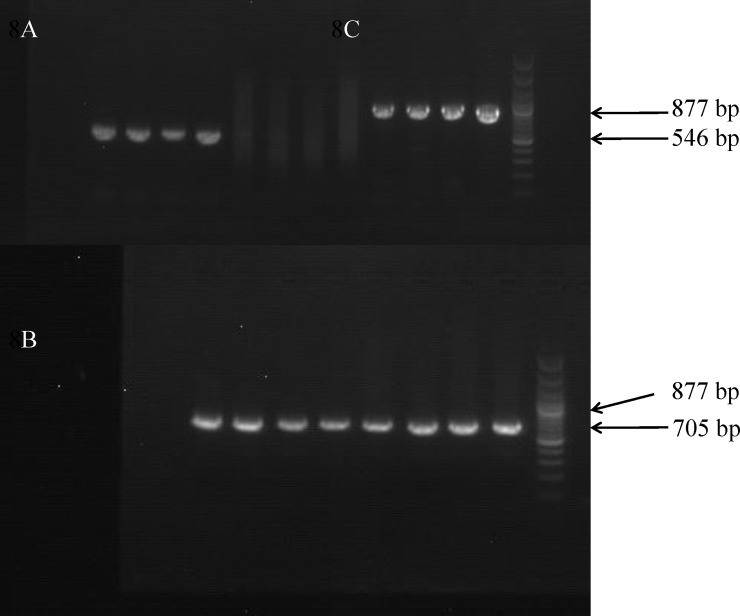
The fragments of PCR amplification for the PON3 gene SNPs are shown in Fig 2. The electrophoresis results showed that the extraction products of rs11767787 (546 bp), rs13226149 (705 bp), and rs17882539 (877 bp) obtained a single band with an appropriate position and clear background.
Fig 2. The electrophoresis of PON3 PCR amplicons for gene.
(A) rs11767787, (B) rs13226149 (C) rs17882539.
Hardy-Weinberg equilibrium
The genotype information for the three SNPs of the PON3 gene in the control population are displayed in Table 4. All of the observed genotype frequencies were in agreement with the HWE (p > 0.05).
Table 4. The HWE for PON3 polymorphisms in control population.
| SNPs | Genotypes | Observed | Theoretical | P value |
|---|---|---|---|---|
| rs11767787 | TT | 115 | 117.04 | 0.106 |
| CT | 35 | 30.92 | ||
| CC | 0 | 2.04 | ||
| rs13226149 | GG | 118 | 119.71 | 0.144 |
| AG | 32 | 28.59 | ||
| AA | 0 | 1.70 | ||
| rs17882539 | CC | 120 | 121.50 | 0.174 |
| CT | 30 | 27 | ||
| TT | 0 | 1.5 |
SNP, single nucleotide polymorphism.
Association of ONID with PON3 SNPs
Only two genotypes were detected for three PON3 SNPs (rs11767787, rs13226149, and rs17882539) in our study population. The genotypes and alleles are shown in Table 5. There were significant differences between the ONID and control groups in the distributions of genotypes and alleles for both rs11767787, rs13226149 and rs17882539 (p < 0.05).
Table 5. Associations of ONID risk with genotype and allele distributions of PON3 polymorphisms.
| SNPs | Models | Total (n = 300) | ONID group | Control group (n = 150) | P value | Crude OR | Adjusted OR* |
|---|---|---|---|---|---|---|---|
| (n = 150) | (95% CI) | (95% CI) | |||||
| rs11767787 | Genotype | 0.013 | |||||
| TT | 211(70.33) | 96(64) | 115(76.67) | 1 | 1 | ||
| CT | 89(29.67) | 54(36) | 35(23.33) | 2.12(1.19–3.77 | 2.12(1.18–3.84) | ||
| Allele | 0.013 | ||||||
| T | 511(85.17) | 246(82) | 265(88.33) | 1 | 1 | ||
| C | 89(14.83) | 54(18) | 35(11.67) | 1.65(1.05–2.6) | 1.68(1.06–2.66) | ||
| rs13226149 | Genotype | 0.003 | |||||
| GG | 211(70.33) | 93(62) | 118(78.67) | 1 | 1 | ||
| AG | 89(29.67) | 57(38) | 32(21.33) | 2.09(1.27–3.43) | 2.09(1.25–3.47) | ||
| Allele | 0.003 | ||||||
| G | 511(85.17) | 243(81) | 268(89.33) | 1 | |||
| A | 89(14.83) | 57(19) | 32(10.67) | 1.86(1.19–2.91) | 1.87(1.19–2.93) | ||
| rs17882539 | Genotype | 0.0009 | |||||
| CC | 215(71.67) | 95(63.33) | 120(80) | 1 | 1 | ||
| CT | 85(28.33) | 55(36.67) | 30(20) | 2.56(1.44–4.57) | 2.59(1.44–4.67) | ||
| Allele | 0.0009 | ||||||
| C | 515(85.83) | 245(81.67) | 270(90) | 1 | |||
| T | 85(14.17) | 55(18.33) | 30(10) | 1.97(1.23–3.16) | 1.95(1.22–3.14) |
The numbers in the parentheses are % in columns 3, 4, and 5.
SNP, single nucleotide polymorphism; ONID, occupational noise-induced deafness; OR, odds ratio; 95% CI, 95% confidence interval.
*Adjusted for age, smoking, drinking, BMI, exposure time and noise exposure level in the conditional logistic regression model.
For rs11767787, there was an enrichment of CT (36%) genotype and allele C (18%) in the ONID cases, and a higher proportion of TT (76.67%) in the control group than in the ONID group (p = 0.013). After adjusting for confounding factors of age, smoking, drinking, BMI, exposure time, and noise exposure level in the conditional logistic regression model, there was a significant association between genotype CT and ONID compared with TT (OR = 2.12, 95% CI: 1.18–3.84, p = 0.01). Meanwhile, allele C was found to be a risk factor for ONID after adjusting for the confounders mentioned above (OR = 1.68, 95% CI: 1.06–2.66, p = 0.03).
The AG genotypes and allele A of rs13226149 were significantly more predominant in the ONID cases than in the control group (p < 0.05). The multivariate analysis results showed that the risk factors for ONID were genotypes AG and allele A, with OR = 2.09 (95% CI: 1.25–3.47, p = 0.005) and OR = 1.87 (95% CI: 1.19–2.93, p = 0.007), respectively.
For rs17882539, ONID cases carrying the CT genotype or allele T were significantly higher than in the control population (p < 0.05). The multivariate analysis results after adjusting for age, smoking, drinking, BMI, exposure time, and noise exposure level showed that the risk factors for ONID were genotypes CT and allele T, with OR = 2.59 (95% CI: 1.44–4.67, p = 0.001) and OR = 1.95 (95% CI: 1.22–3.14, p = 0.006), respectively.
Characteristics of plasma PON3 activity
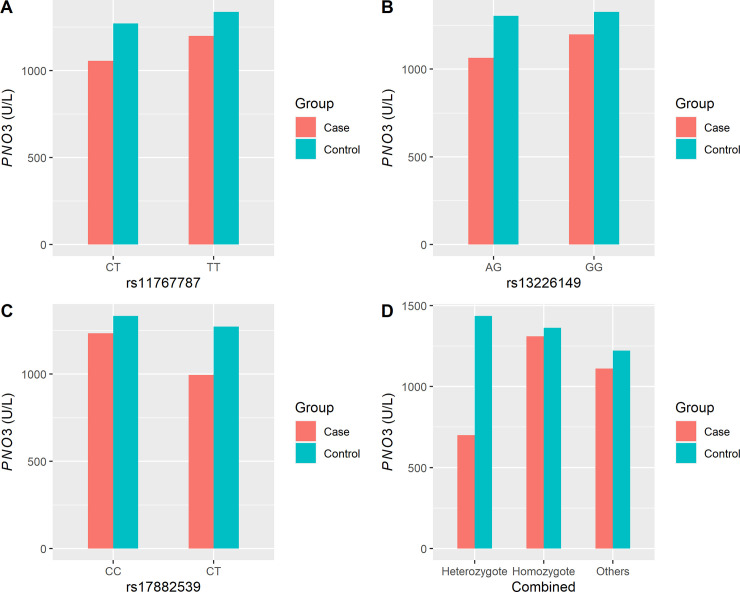
Plasma PON3 activity in the ONID group was 1147.04±379.48 U/L, which was significantly lower than that of the control group (1322.24±374.64 U/L). We then we detected significant differences in the plasma PON3 activity between the three PON3 SNPs rs11767787, rs13226149, and rs17882539 between the ONID and control groups (p < 0.05), except for genotype CC of rs17882539 (Fig 3).
Fig 3. Distributions of plasma PNO3 activity in the ONID and control groups for rs11767787, rs13226149 and rs17882539, and combined, respectively.
Red bars represent control group and blue bars represent ONID cases. The genotypes are indicated below each bar chart. A showed PON3 activity at rs11767787, B showed PON3 activity at rs13226149, C showed PON3 activity at rs17882539, and D showed PON3 activity at three SNPs combined.
Furthermore, all participants were divided into homozygote, heterozygote, and other subgroups. Subjects caring for genotypes of TT, GG, and CC for rs11767787, rs13226149, and rs17882539, respectively, were divided into the homozygous group. Subjects caring genotypes of CT, AG, and CT for rs11767787, rs13226149, and rs17882539, respectively, were divided into the heterozygous group. All others were divided into the other sub-group. There were significant differences in the heterozygous genotype distributions between the ONID and control groups (p < 0.05); however, no significant differences were found in the homozygous and other subgroups between ONID and control population (Fig 3).
Association of plasma PON3 activity with ONID
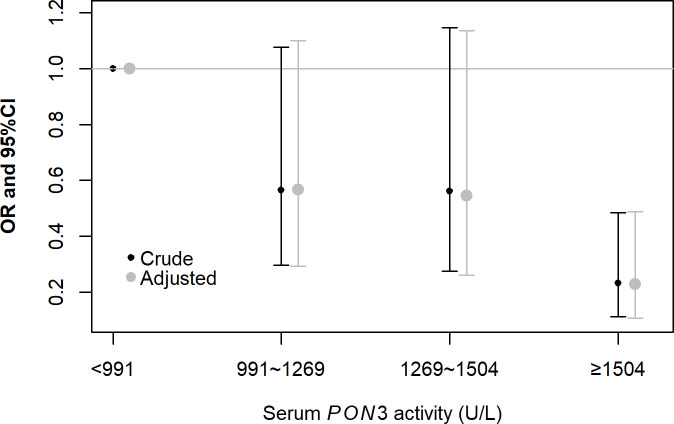
Plasma PNO3 activity was categorized into four groups according to the IQR (Fig 4). In multivariate analysis adjusting for age, smoking, drinking, BMI, exposure time, and noise exposure levels in the conditional logistic regression model, the OR of ONID decreased 0.23-fold (95% CI: 0.11–0.49, p = 0.0001) in the highest quantile category (more than 1504 U/L) compared with the lowest quantile category (less than 991 U/L). However, we did not find statistically significant associations between the other two plasma PNO3 activity categories and ONID in the multivariate analysis (p > 0.05).
Fig 4. The risk of plasma PON3 activity is associated with ONID in four groups.
The exposure plasma PNO3 activity was categorized into four groups (less than P25, P25-P50, P50-P75, greater than P75), considering the lowest group as the reference.
Discussion
To the best of our knowledge, this is the first study on the relationship between the PON3 gene polymorphisms and susceptibility to ONID in industrial workers in China. In this matched case-control study, only two genotypes were isolated from peripheral venous blood samples for PON3 gene SNPs rs11767787, rs13226149, and rs17882539. Significant differences between the ONID and control groups were observed in the distribution of genotypes and alleles for both rs11767787, rs13226149, and rs17882539 (p < 0.05). The independent risk factors for ONID were genotype CT and allele C, AG and allele A and CT and allele T for rs11767787, rs13226149, and rs17882539, respectively, irrespective of age, smoking, drinking, BMI, exposure time, and noise exposure level. Furthermore, the plasma PON3 activity in the ONID group was significantly lower than that of the control group and the same as in different genotypes for three PON3 SNPs (rs11767787, rs13226149, and rs17882539). Plasma PON3 level (> 1504 U/L) was observed to be a protective factor associated with the lowest level of ONID (less than 991 U/L) adjusting for age, smoking, drinking, BMI, exposure time, and noise exposure level.
The development of ONID depends on factors concerning noise such as the duration of exposure and the intensity and frequency of the noise. The individual susceptibility to noise is hypothesized to correlate with hearing loss, including increasing age, male sex, smoking, and alcohol [30–33]. We conducted a matched case-control study to investigate the potential relationship between ONID and PON3 polymorphisms in Shenzhen, and age, gender, and duration of exposure time were matched to the ONID cases to exclude the important confounders. Meanwhile, other confounders such as smoking, drinking, and noise exposure level were also balanced in our study. We found that the ONID group exhibited higher hearing thresholds at frequencies from 0.5 to 6.0 kHz in comparison with the control group. The differences in hearing thresholds between the ONID and control groups at frequency 3.0, 4.0, and 6.0 kHz were observed to be stable, consistent with the occurrence and development of ONID.
Individual susceptibility to noise is another important factor associated with inner ear vulnerability to noise after equivalent noise exposure. The etiology of ONID, which remains an unavoidable problem in industrial workers, involves an interaction between environmental and genetic factors. In fact, studies have shown that the concentrations of superoxide radicals in the cochlear fluid as well as in the stria vascularis increased after exposure to noise in animal models. Thus, genes involved in the regulation of reactive oxygen species, such as antioxidant activity PONs genes, may affect the vulnerability of the cochlea to ONID [16]. All PON genes are antioxidants and can hydrolyze a variety of substrates. There are several positive associations between the PON2 gene and NIHL populations in Italy [34] and the Chinese population [17]. The association of PON3 gene with ONID has, to our knowledge, not been investigated in a single comprehensive data set. Our data showed that the allelic variants for SNP rs11767787, rs13226149, and rs17882539 were rare (frequency < 1%) in this population. No homozygous (CC, AA, and TT) individuals for these mutations were identified. However, the results still found that the heterozygous alleles C, A, and T for SNP rs11767787, rs13226149, and rs17882539 were all associated with OIND, suggesting that individuals carrying the alleles C, A, and T may be more prone to ONID when exposed to occupational noise. The exact mechanisms involved remain unknown, however, warrant further investigation.
We also evaluated the effect of the variant on the metabolic activity of the PON3 gene. PON1 and PON3 are found in the serum, whereas PON2 is primarily an intracellular enzyme [35]. Our results suggest that low plasma PON3 activity is a risk factor for OIND, especially for individuals with less than 991 U/L. Furthermore, we also found that plasma activities in the heterozygous ONID cases were significantly lower than in the control population for rs11767787, rs13226149 and rs17882539, and combined. Meanwhile, the levels of plasma PON3 activity in the homozygous group were higher than in the heterozygous group in both ONID cases and the control population for the three SNPs. However, a slightly higher level of PNO3 activity was found in the heterozygous subgroup compared the that of the homozygous subgroup for SNPs combined. This may be biased by the small sample size, only nine controls contained the heterozygote for the three SNPs together. Thus, further research is needed to illustrate the relationship between the genotype and PNO3 activity. Our findings suggested that the PON3 variants could strongly influence PON3 activity, and that the overproduction heterozygous genotype may be capable of lowering the antioxidant activity. Serum PON activity is reduced in patients with Alzheimer’s disease [36] and systemic lupus erythematosus [37], which was consistent with our results.
There are some limitations to our study. First, the case-control study did not explain the causal relationship, whether the ONID was caused by PON3 variants or vice versa. Second, the research subjects were all Chinese. Due to ethnic differences, more studies are needed to better understand the associations of PON3 with the risk of developing ONID. Third, the sample size was small in our study; the homozygotes CC, AA, and TT for SNP rs11767787, rs13226149, and rs17882539 were not identified. A larger sample size and comprehensive resequencing may help to better understand the association analysis of the PON3 gene with the risk of ONID in future work.
Conclusions
In conclusion, we suggest that the PON3 variants, rs11767787 C allele, rs13226149 A allele, and rs17882539 T allele and their associated plasma enzyme activities may increase the risk of developing ONID in Chinese industrial workers independent of age, smoking, drinking, BMI, exposure time, and noise exposure level. We also found that PON3 variants strongly influenced the PON3 activity and may be potential biomarkers for detecting ONID in noise-exposed workers.
Supporting information
Demographic characteristics and genotype result of each subject.
(CSV)
(DOCX)
(DOCX)
(DOCX)
(PDF)
(TIF)
(JPG)
Acknowledgments
We thank all noise-exposed workers who participated in the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Huaping Zhou was funded by Shenzhen Health and Family Planning Commission research project (SZFZ2017023). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tessier-Sherman B, Galusha D, Cantley LF, Cullen MR, Rabinowitz PM, Neitzel RL. Occupational noise exposure and risk of hypertension in an industrial workforce. Am J Ind Med. 2017;60(12):1031–1038. 10.1002/ajim.22775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Münzel T, Steven S, Frenis K, Lelieveld J, Hahad O, Daiber A. Environmental Factors Such as Noise and Air Pollution and Vascular Disease. Antioxid Redox Signal. 2020. 10.1089/ars.2020.8090 [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Han L, Dai S, Li X, Cai W, Yang D, et al. Hearing Loss Characteristics of Workers with Hypertension Exposed to Occupational Noise: A Cross-Sectional Study of 270,033 Participants. Biomed Res Int. 2018;2018:8541638 10.1155/2018/8541638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Institute for Health Metrics and Evaluation. Global Health Data Exchange 2017. Available from: http://ghdx.healthdata.org/gbd-results-tool (accessed on 13 June 2020).
- 5.Miao L, Ji J, Wan L, Zhang J, Yin L, Pu Y. An overview of research trends and genetic polymorphisms for noise-induced hearing loss from 2009 to 2018. Environ Sci Pollut Res Int. 2019;26(34):34754–34774. 10.1007/s11356-019-06470-7 [DOI] [PubMed] [Google Scholar]
- 6.Hu L, Deng JH. Progress in the association studies on susceptibility gene SNP for NIHL in human population. Occup and Health. 2014;30(18):2664–2669. [Google Scholar]
- 7.Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4(5):229–236. 10.1159/000013846 [DOI] [PubMed] [Google Scholar]
- 8.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, et al. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21(4):542–547. 10.1161/01.atv.21.4.542 [DOI] [PubMed] [Google Scholar]
- 9.Shen H, Huo X, Liu K, Li X, Gong W, Zhang H, et al. Genetic variation in GSTM1 is associated with susceptibility to noise-induced hearing loss in a Chinese population. J Occup Environ Med. 2012;54(9):1157–1162. 10.1097/JOM.0b013e31825902ce [DOI] [PubMed] [Google Scholar]
- 10.Barranco I, Roca J, Tvarijonaviciute A, Ruber M, Vicente-Carrillo A, Atikuzzaman M, et al. Measurement of activity and concentration of paraoxonase 1 (PON-1) in seminal plasma and identification of PON-2 in the sperm of boar ejaculates. Mol Reprod Dev. 2015;82(1):58–65. 10.1002/mrd.22444 [DOI] [PubMed] [Google Scholar]
- 11.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33(3):498–507. 10.1006/geno.1996.0225 [DOI] [PubMed] [Google Scholar]
- 12.Nie Y, Luo D, Yang M, Wang Y, Xiong L, Gao L, et al. A Meta-Analysis on the Relationship of the PON Genes and Alzheimer Disease. J Geriatr Psychiatry Neurol. 2017;30(6):303–310. 10.1177/0891988717731825 [DOI] [PubMed] [Google Scholar]
- 13.Lioudaki S, Verikokos C, Kouraklis G, Ioannou C, Chatziioannou E, Perrea D, et al. Paraoxonase-1: Characteristics and Role in Atherosclerosis and Carotid Artery Disease. Curr Vasc Pharmacol. 2019;17(2):141–146. 10.2174/1570161115666171129212359 [DOI] [PubMed] [Google Scholar]
- 14.Liu YL, Yang J, Zheng J, Liu DW, Liu T, Wang JM, et al. Paraoxonase 1 polymorphisms L55M and Q192R were not risk factors for Parkinson's disease: a HuGE review and meta-analysis. Gene. 2012;501(2):188–192. 10.1016/j.gene.2012.03.067 [DOI] [PubMed] [Google Scholar]
- 15.Li XT, Li X, Hu FF, Shen HX, Cao JL, Zhong L, et al. Association between Paraoxonase 2 Gene Polymorphisms and Noise-induced Hearing Loss in the Chinese Population. J Occup Health. 2013;55(2):56–65. WOS:000323467300002 10.1539/joh.12-0242-oa [DOI] [PubMed] [Google Scholar]
- 16.Fortunato G, Marciano E, Zarrilli F, Mazzaccara C, Intrieri M, Calcagno G, et al. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem. 2004;50(11):2012–2018. 10.1373/clinchem.2004.037788 [DOI] [PubMed] [Google Scholar]
- 17.Cao JL, Li XT, Zhong L, Shen HX, Ding L, liu J, et al. Association between single nucleotide polymorphisms of PON2 gene and susceptibility to occupational noise-induced deafness among Chinese Han population exposed to high noise levels. Chin J Ind Hyg Occup Dis. 2013;31(10):734–739. [PubMed] [Google Scholar]
- 18.Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(1):78–88. 10.1007/s00210-003-0833-1 [DOI] [PubMed] [Google Scholar]
- 19.Campo S, Sardo AM, Campo GM, Avenoso A, Castaldo M, D'Ascola A, et al. Identification of paraoxonase 3 gene (PON3) missense mutations in a population of southern Italy. Mutat Res. 2004;546(1–2):75–80. 10.1016/j.mrfmmm.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 20.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46(6):1239–1247. 10.1194/jlr.M400511-JLR200 [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Peng W, Jiang X, Chen B, Zhu J, Zang Y, et al. Transgene expression of human PON1 Q in mice protected the liver against CCl4-induced injury. J Gene Med. 2008;10(1):94–100. 10.1002/jgm.1128 [DOI] [PubMed] [Google Scholar]
- 22.Rull A, García R, Fernández-Sender L, García-Heredia A, Aragonès G, Beltrán-Debón R, et al. Serum paraoxonase-3 concentration is associated with insulin sensitivity in peripheral artery disease and with inflammation in coronary artery disease. Atherosclerosis. 2012;220(2):545–551. 10.1016/j.atherosclerosis.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 23.García-Heredia A, Marsillach J, Aragonès G, Guardiola M, Rull A, Beltrán-Debón R, et al. Serum paraoxonase-3 concentration is associated with the severity of hepatic impairment in patients with chronic liver disease. Clin Biochem. 2011;44(16):1320–1324. 10.1016/j.clinbiochem.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Alafris A, Longo AJ, Cohen H. Irreversible atorvastatin-associated hearing loss. Pharmacotherapy. 2012;32(2):e27–34. 10.1002/PHAR.1040 [DOI] [PubMed] [Google Scholar]
- 25.Riedmaier S, Klein K, Winter S, Hofmann U, Schwab M, Zanger UM. Paraoxonase (PON1 and PON3) Polymorphisms: Impact on Liver Expression and Atorvastatin-Lactone Hydrolysis. Front Pharmacol. 2011;2:41 10.3389/fphar.2011.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shenzheng China. Shenzheng statistical yearbook 2019. Available from: http://www.sz.gov.cn/cn/xxgk/zfxxgj/tjsj/tjnj/content/post_6616865.html (accessed on 13 June 2020).
- 27.National Health Commission of the People's Republic of China. Diagnosis of occupational noise-induced deafness (GBZ 49–2014). 2014. Available from: http://www.nhc.gov.cn/wjw/pyl/201410/12e4ec65af8e46248bb45d366a0d5021.shtml (accessed on 1 June 2020).
- 28.National Health Commission of the People's Republic of China. Measurement of Noise in the Workplace (GBZ 189.9–2007). 2007. Available from: http://www.nhc.gov.cn/ewebeditor/uploadfile/2014/10/20141030103830863.pdf (accessed on 1 June 2020).
- 29.Sangon Biotech.2019. Available from: https://www.sangon.com/productImage/DOC/B518253/B518253_ZH_P.pdf (accessed on 15 July 2020).
- 30.Le TN, Straatman LV, Lea J, Westerberg B. Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg. 2017;46(1):41 10.1186/s40463-017-0219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovalova M, Mrazkova E, Sachova P, Vojkovska K, Tomaskova H, Janoutova J, et al. Hearing Loss in Persons Exposed and not Exposed to Occupational Noise. J Int Adv Otol. 2016;12(1):49–54. 10.5152/iao.2016.1770 [DOI] [PubMed] [Google Scholar]
- 32.Curhan SG, Eavey R, Wang M, Stampfer MJ, Curhan GC. Prospective study of alcohol consumption and self-reported hearing loss in women. Alcohol. 2015;49(1):71–77. 10.1016/j.alcohol.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrite S, Santana V. Joint effects of smoking, noise exposure and age on hearing loss. Occup Med (Lond). 2005;55(1):48–53. 10.1093/occmed/kqi002 [DOI] [PubMed] [Google Scholar]
- 34.Fortunato G, Marciano E, Zarrilli F, Mazzaccara C, Intrieri M, Calcagno G, et al. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem. 2004;50(11):2012–2018. 10.1373/clinchem.2004.037788 WOS:000224717500012 10.1373/clinchem.2004.037788 [DOI] [PubMed] [Google Scholar]
- 35.Stoltz DA, Ozer EA, Recker TJ, Estin M, Yang X, Shih DM, et al. A common mutation in paraoxonase-2 results in impaired lactonase activity. J Biol Chem. 2009;284(51):35564–35571. 10.1074/jbc.M109.051706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erlich PM, Lunetta KL, Cupples LA, Abraham CR, Green RC, Baldwin CT, et al. Serum paraoxonase activity is associated with variants in the PON gene cluster and risk of Alzheimer disease. Neurobiol Aging. 2012;33(5):1015.e1017–1023. 10.1016/j.neurobiolaging.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiss E, Seres I, Tarr T, Kocsis Z, Szegedi G, Paragh G. Reduced paraoxonase1 activity is a risk for atherosclerosis in patients with systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1108:83–91. 10.1196/annals.1422.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic characteristics and genotype result of each subject.
(CSV)
(DOCX)
(DOCX)
(DOCX)
(PDF)
(TIF)
(JPG)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.